Cap Cancer Template - CAP Cancer Protocol Templates provide general for collecting the essential data elements for complete reporting of malignant tumors furthermore optimum patient care
The electronic versions of over 100 Cancer Protocols and Cancer Biomarker Templates help pathologists create high quality cancer reports within their current system workflows
Cap Cancer Template

Cap Cancer Template
CAP Cancer Protocol Style provide mission for collecting the basic data elements for complete reporting a malignant tumors and optimal patient… CAP Cancer Protocol Templates provide guidelines for collecting the essential data elements for complete reporting of malignant carcinomas and optimal patient care.
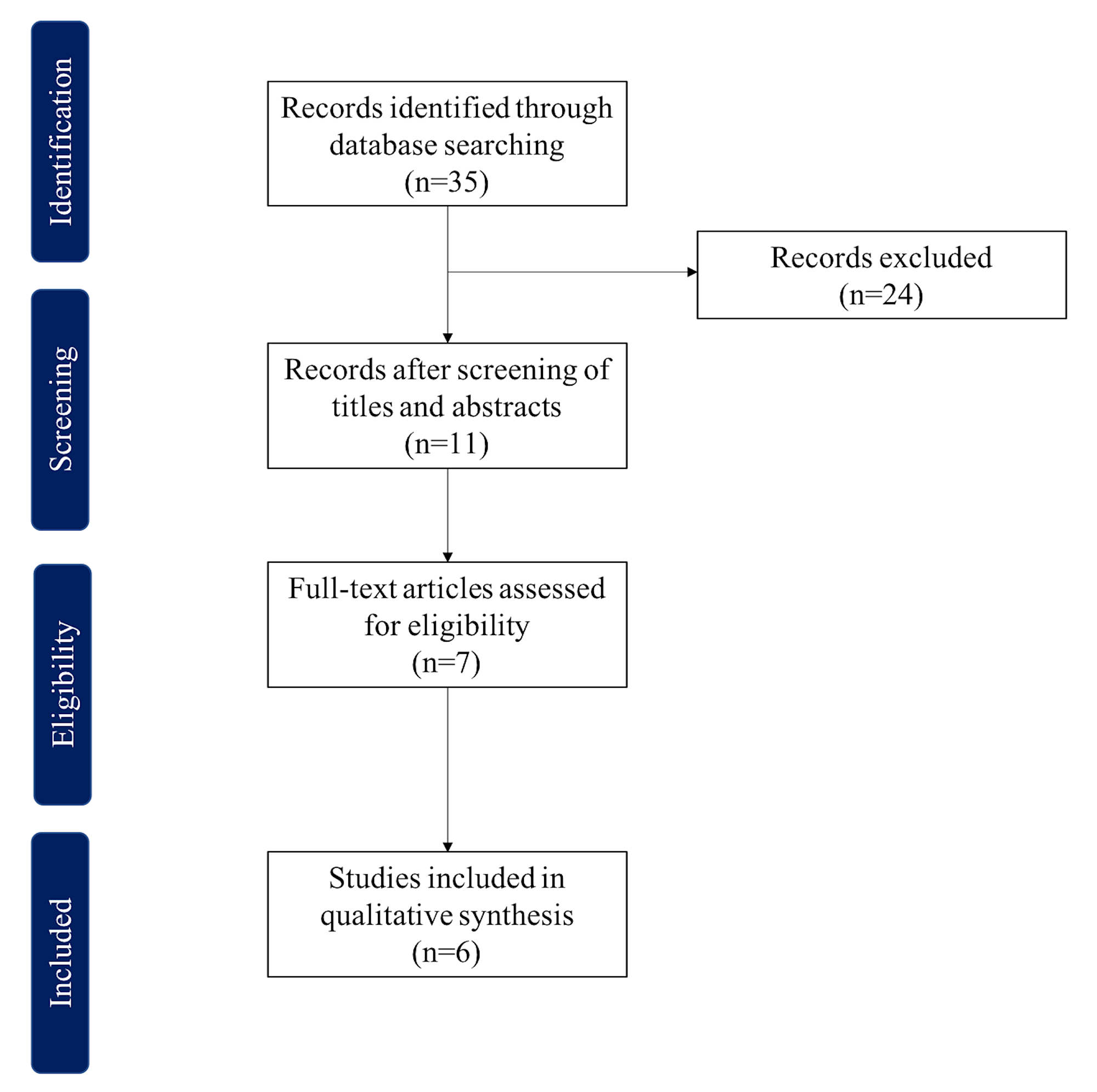
Current Cancer Protocols View current protocols and download templates Cancer Protocols Units of Measure Read more about the CAP Notice regarding Units of Measurement in the CAP electronic Cancer Checklists eCP and CAP Cancer Protocols Cancer Protocol Review Process Learn how the CAP develops protocols and conducts
Cancer Reporting Tools College Of American Pathologists
What are the Cancer Biomarker Reporting Templates Is use of the cancer biomarker reporting templates required by accreditation If hormone receptor and HER2 testing is performed on a previous breast biopsy specimen do those results need to be included in the breast resection pathology report
Cap Cancer Protocol Mesothelioma
Cancer biomarker testing results the CAP Colorectal Biomarker Template should be used Pending biomarker studies should be listed in the Comments section of this report

Pathology Template With CAP Explanatory Notes For CAP Kidney Protocol

Cap Cancer Template
Cancer Protocol Templates College Of American Pathologists
For reporting molecular testing and immunohistochemistry for mismatch repair proteins and for other cancer biomarker testing results the CAP Colorectal Biomarker Template should be used Pending biomarker studies should be listed in the Comments section of this report

CAP Explanatory Notes For Endometrium Carcinoma Embedded In An EMR
Our Cancer Reporting Protocols are used by thousands of pathologists and other medical professionals to provide complete and uniform reporting of malignant tumors Printable versions of the standards in Word or PDF formats are available for free
This protocol can be utilizedfor a variety of procedures and tumor types for clinical care purposes. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format.
Cancer Protocol Templates College Of American Pathologists
The CAP releases new and updated electronic Cancer Protocols eCP templates on a rolling basis coordinating as much as possible with the posting of new and revised Cancer Protocols and Cancer Biomarker Reporting Templates
Cap Cancer Template

Chemo Cap Cancer Beanie Sleep Casual Light Gray All Etsy
Cap Cancer Template
Our Cancer Reporting Protocols are used by thousands of pathologists and other medical professionals to provide complete and uniform reporting of malignant tumors Printable versions of the standards in Word or PDF formats are available for free
The electronic versions of over 100 Cancer Protocols and Cancer Biomarker Templates help pathologists create high quality cancer reports within their current system workflows

Explore Breast Cancer Google Slides Template Design
Free Cap Template FREE PRINTABLE TEMPLATES

Fragment Of CAP Cancer Checklist For Melanoma Fragment Of CAP Cancer

CT Lung Screening Form
![]()
How To Extend CAP Cancer Protocols For Clinical Research Softworks Group