Enclinical Evaluation Report Template Mdr - 5 Clinical evaluation under EU MDR 2 Purpose of a clinical evaluation Article 61 1 of the EU MDR indicates that manufacturers shall plan conduct and document a clinical evaluation
A Clinical Evaluation Report CER is a complex technical document that summarises the process of Clinical Evaluation a component of all medical device regulatory submissions under the MDR The introduction of the Medical Device Regulation EU 2017 745 means that Clinical Evaluation Report writing will need to be completed to a higher
Enclinical Evaluation Report Template Mdr

Enclinical Evaluation Report Template Mdr
The Clinical Evaluation Report - CER is the document which contains the results of the - application of the clinical evaluation requirements and process by the manufacturer, for a medical device. The MDR and also current MEDDEV 2.7.1 Rev 4 - Clinical Evaluation contains general information on the contents of the CER (the MEDDEV also provides ...
The Medical Device Regulation MDR applies from 26 May 2021 The In Vitro Diagnostics Regulation IVDR applies from the 22 May 2022 These dates may shift depending on delays This is a medical
Clinical Evaluation Report CER Templates For MDR Compliance
WHAT TO EXPECT IN THIS 4 PART SERIES Part 1 Clinical Evaluation of a Medical Device Creating a Process and Establishing Equivalency Part 2 The Clinical Evaluation Literature Review Process Identifying and Appraising Clinical Data Part 3 Performing Data Analysis for Your Medical Device s Clinical Evaluation

Medical Evaluation Report Templates At Pertaining To Template For
ClinicalEvaluationReport TheClinicalEvaluationReportstatestheclinicalbenefitsandsafetycharac teristicsofthedevice basedonclinicaldata ItistheoutputoftheClinical

Evaluation Report Template Docedeportes

Medical Evaluation Report How To Create A Medical Evaluation Report
Span Class Result Type
Girish Hirpara regulatory consultant on Kolabtree provides a clinical evaluation report sample for medical devices to use as a template for MDR compliance The clinical evaluation report CER is a mandatory document for medical devices that are to be placed in the EU market The CER is submitted along with the technical file to meet EU MDR requirements

14 Types Of Reports And When To Use Them Templates
Your complete EU MDR Compliance Toolkit Edition 4 0 Free sample Produce EU MDR compliant CERs for any class of medical device using a proven start to nish methodology
Clinical Evaluation Report Created: Daniela Penn Revision/Version: 1.0 Date: 20.11.2020 File: Clinical-Evaluation-Report-MBST_V1. Applicable SOP: CE-SOP-01 (medXteam GmbH) Page 1/112
Span Class Result Type
The clinical evaluation plan necessary for creating the CER is detailed in paragraph 1 of Part A of Annex 14 This plan consists of the following stages Stage 0 Planning stage to create the clinical evaluation plan Stage 1 Defining relevant clinical data Stage 2 Appraising the clinical data Stage 3 Either analysing the

Fillable Online Clinical Evaluation Plan Report Fax Email Print PdfFiller
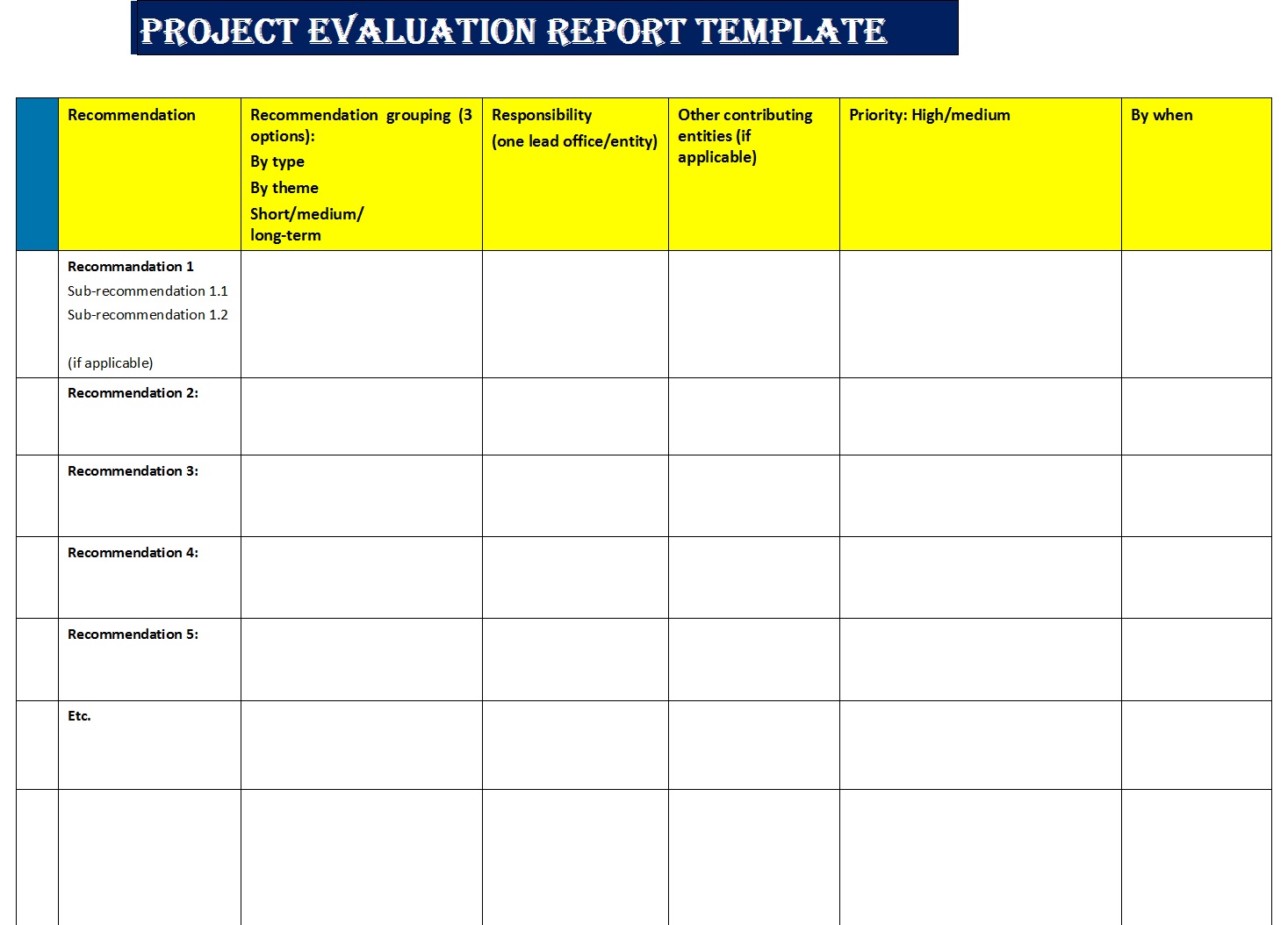
Project Evaluation Report Template Free Report Templates
Enclinical Evaluation Report Template Mdr
Your complete EU MDR Compliance Toolkit Edition 4 0 Free sample Produce EU MDR compliant CERs for any class of medical device using a proven start to nish methodology
A Clinical Evaluation Report CER is a complex technical document that summarises the process of Clinical Evaluation a component of all medical device regulatory submissions under the MDR The introduction of the Medical Device Regulation EU 2017 745 means that Clinical Evaluation Report writing will need to be completed to a higher

Clinical Evaluation Report Template Mdr
How To Publish Evaluation Report Card On Parent Portal And Parent

Evaluation Report Template Docedeportes

Standard Evaluation Report How To Create A Standard Evaluation Report
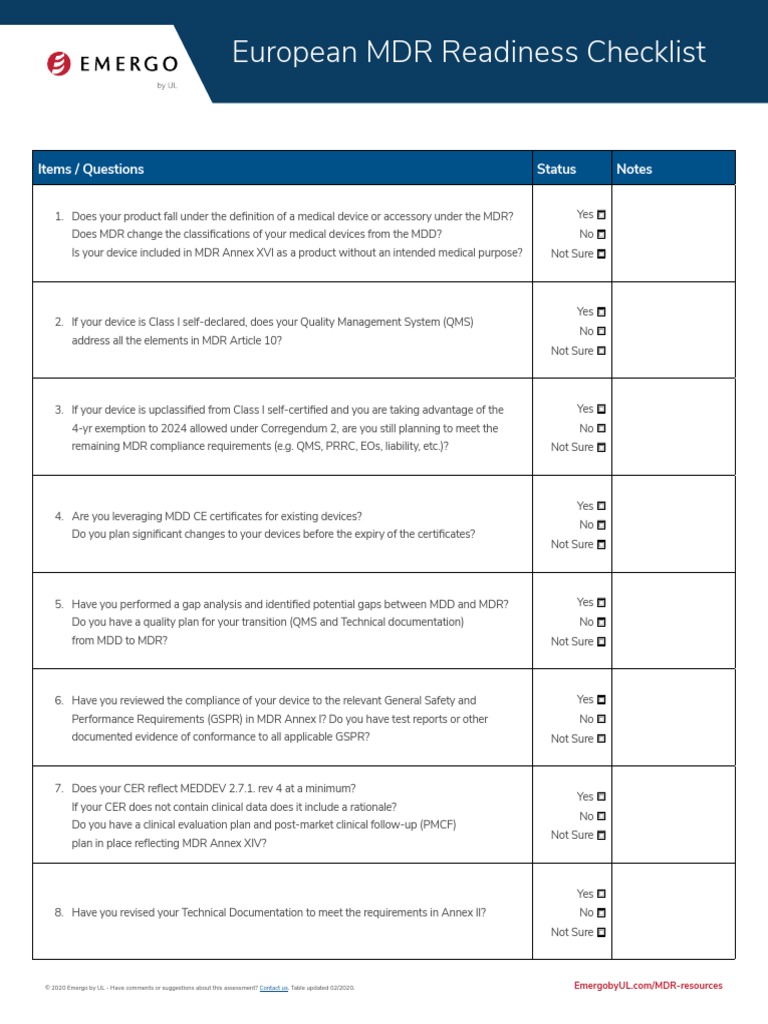
European MDR Readiness Checklist Fillable PDF Quality Management