Enclinical Trial Risk Management Plan Template - Welcome to Global Health Trials tools and templates library Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added
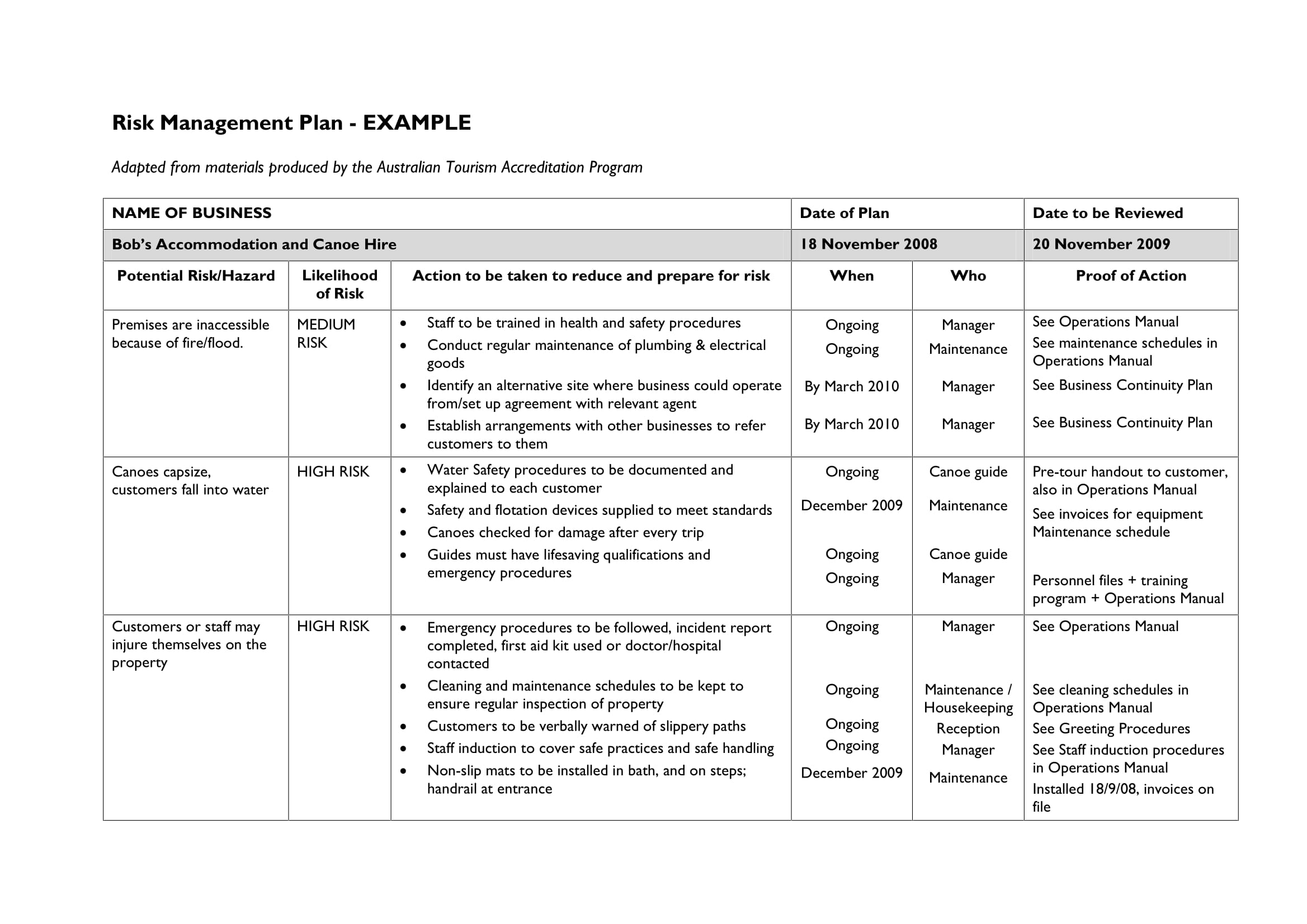
RISK MANAGEMENT PLAN FOR RESEARCH ON THE ALFRED CAMPUS 1 The context The Alfred campus is one of Australia s leading centres in clinical and biomedical research Several of the campus research groups are regarded as international leaders Research is a core activity of all the institutions on The Alfred campus
Enclinical Trial Risk Management Plan Template
Enclinical Trial Risk Management Plan Template
Sponsors (i.e. Chief/Principal Investigator for i nvestigator-initiated trials ) should implement a risk assessment and management plan to manage quality throughout all stages of the trial from trial design through to
1 Identify main risks associated with the clinical trial Classify risks based on their severity and probability Prepare risk mitigation strategies for each identified risk Draft the initial Risk Management Plan document Approval Risk Management Plan by Project Manager Develop a plan for continuous risk assessment and management
Span Class Result Type
Risk management in clinical trials is an ever changing and improving process that s all the more critical with the increases in trial complexity and reductions in R D budgets As trials grow so do the principles of thought and action that go into mitigating risks in trial design and execution This piece covers the current state of risk
Risk Management Plan Template Edit Fill Sign Online Handypdf
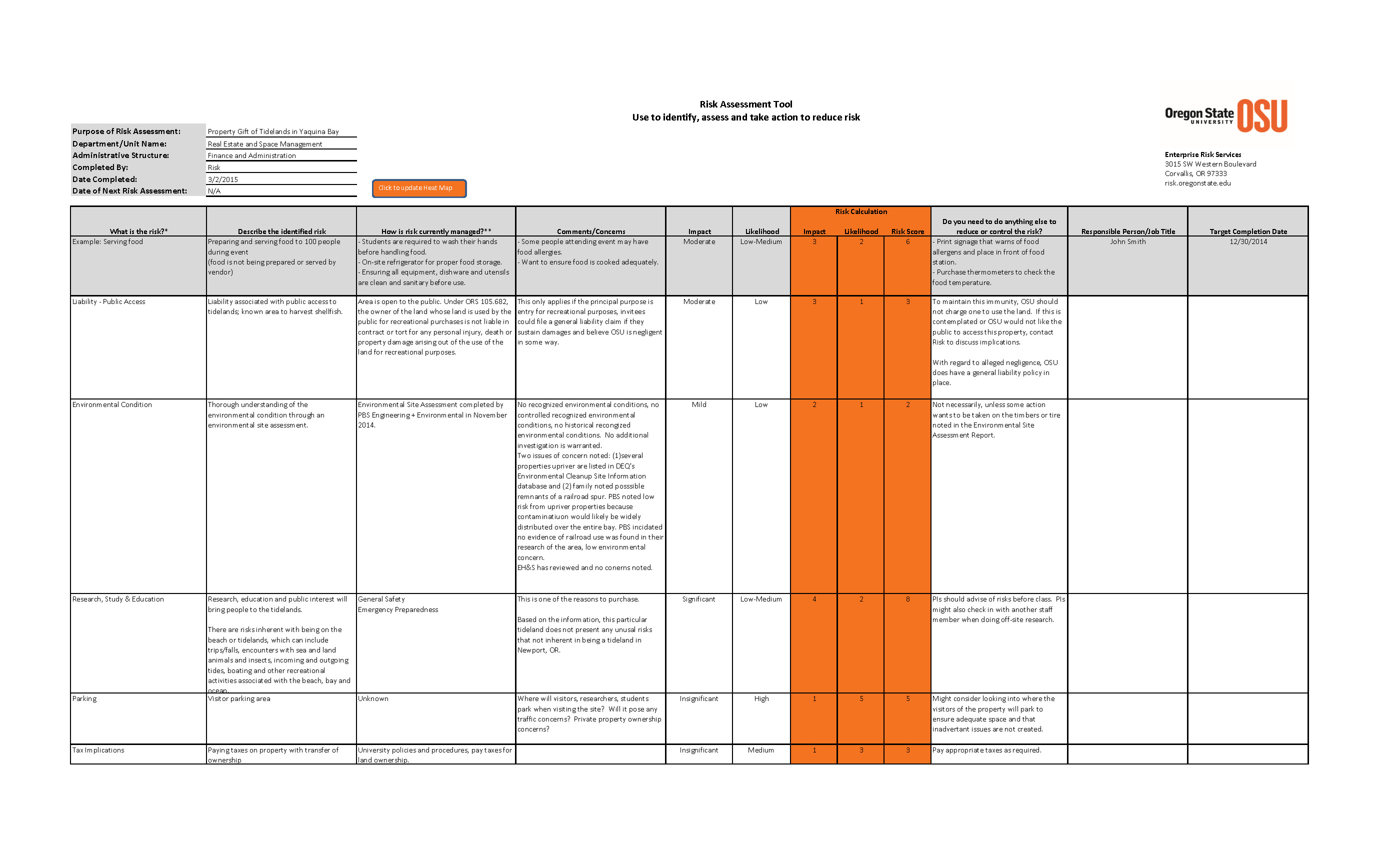
The purpose of this CTIS Risk Mitigation Plan is to strengthen planning for the functioning of the CTIS in order to ensure that business as usual activities can be performed by users within the system This is done through a continuous monitoring of operations in line with the CTIS programme governance

Sample Example Format Templates 11 Sample Risk Mitigation Plan
Free Risk Assessment Spreadsheet Machine Safety Specialists Riset
Downloadable Templates And Tools For Clinical Research
Introduction Clinical trials are conventionally monitored by source data verification that is costly requires ample resources and exhibits several limitations 1 2 The International Council for Harmonization ICH has provided sponsors with the flexibility to initiate a novel approach called risk based monitoring RBM to enhance quality management in a clinical trial 3 Regulatory
Clinical Trial Risk Management Plan Ppt Powerpoint Presentation Outline
A Clinical Trial Risk Management Plan outlines how to manage risks in a clinical trial because it serves as the basis for all other risk management activities including risk identification assessment mitigation and monitoring Cyntegrity s Clinical Trial Risk Management Plan Tool helps you save time and effort because it provides
Multi-site Appendix G-1: Demographics Form. Multi-site Appendix G-2: Medical History Form. Multi-site Appendix G-3: Prior and Concomitant Medications Form. Multi-site Appendix G-4: Vital Signs Form. Multi-site Appendix G-5: Study Disposition Form. Multi-site Appendix H: Sample Clinical Trial Closeout Procedures.
Span Class Result Type
Here we present results from the third annual survey which included data from 4889 clinical trials ongoing in 2021 At least one RBQM component was implemented in 88 of trials in the 2021 survey compared with 77 in 2020 and 53 in 2019 The most frequently implemented components in 2021 were initial and ongoing risk assessments 80 and 78
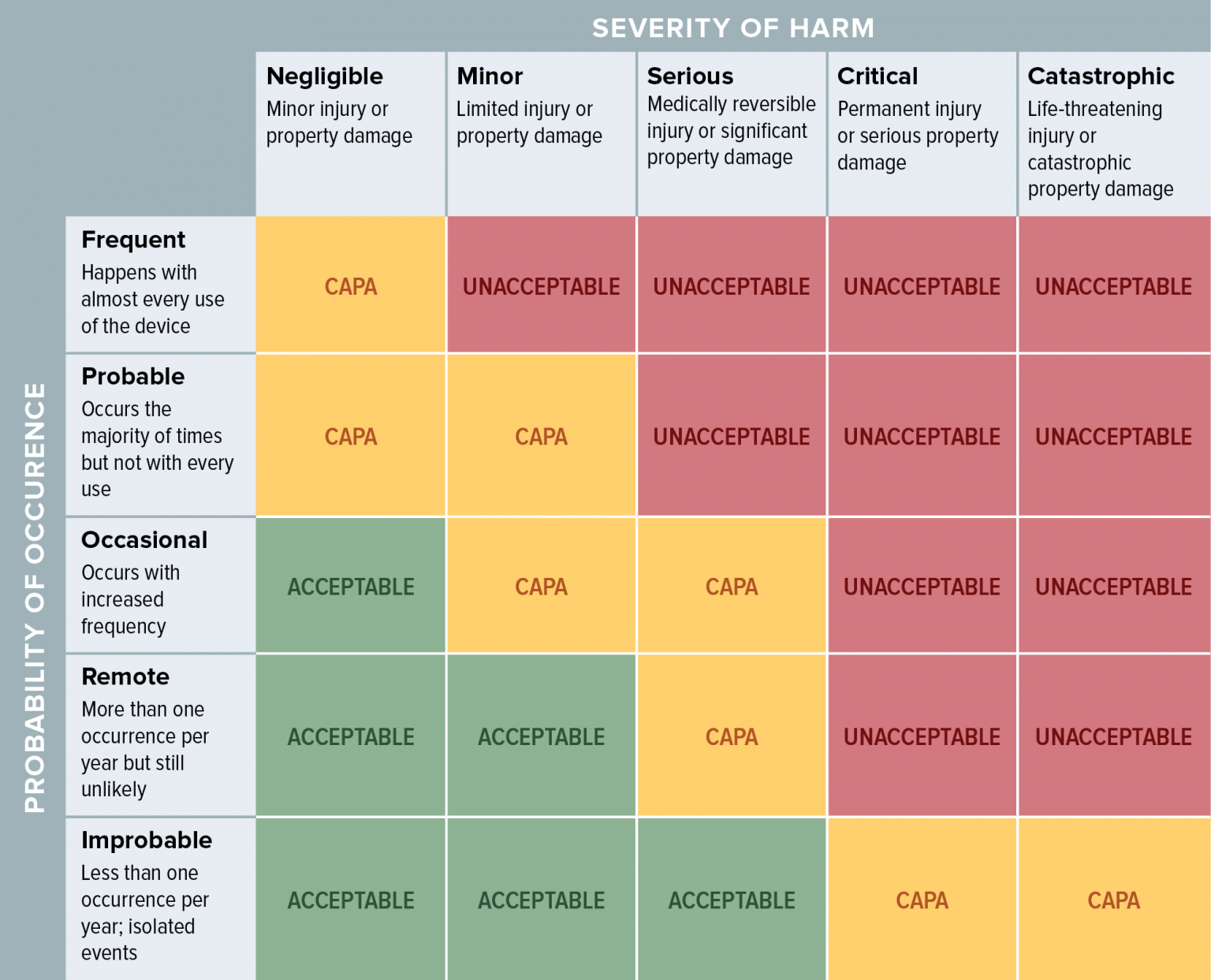
Creating A Medical Device Risk Management Plan And Doing Analysis

Risk Management Plan Template ASC A2 It Is Part Of The Supporting
Enclinical Trial Risk Management Plan Template
A Clinical Trial Risk Management Plan outlines how to manage risks in a clinical trial because it serves as the basis for all other risk management activities including risk identification assessment mitigation and monitoring Cyntegrity s Clinical Trial Risk Management Plan Tool helps you save time and effort because it provides
RISK MANAGEMENT PLAN FOR RESEARCH ON THE ALFRED CAMPUS 1 The context The Alfred campus is one of Australia s leading centres in clinical and biomedical research Several of the campus research groups are regarded as international leaders Research is a core activity of all the institutions on The Alfred campus

Pin On Bluesafe Solution

Risk Chart Template All Business Templates

Trial Report Template

Risk Management Report Template Examples Iso 14971 Medical Inside

Best Risk Management Plan Template Excel Format In 2021 Risk