Endata Validation Plan Template - VMPs must be written in a concise to the point and comprehensible manner Write as if the reader has no prior knowledge this will result in simple specific terms Always write in active voice using present tense verbs and never give the reader a choice by using words like may or should
It is a document that describes the overall strategy for validating a process or system Organizations use it to ensure that their processes and systems comply with quality safety and regulatory standards It outlines the validation activities objectives scope approach and responsibilities
Endata Validation Plan Template

Endata Validation Plan Template
2. The Validation Master Plan (VMP) is a summary of the planned validation activities. It lists those activities and essential documents which will be generated and defines staff responsibilities. As it is a summary, it does not repeat information documented in validation protocols or standard operating procedures.
Data Validation Plan DVP Edit Check EC Test Case Semi automatic DVP Tool SatDVP Data Manager DM Database Designer DBD INTRODUCTION As a traditional process in clinical data management DVP writing usually cost quite much data manager time and communication Only a data manager with enough experience and well mutual understanding
What Is Validation Master Plan Template Examples ERP Information
The purpose of the V V Plan is to identify the activities that will establish compliance with the requirements verification and to establish that the system will meet the customers expectations validation 1 2 Responsibility and Change Authority
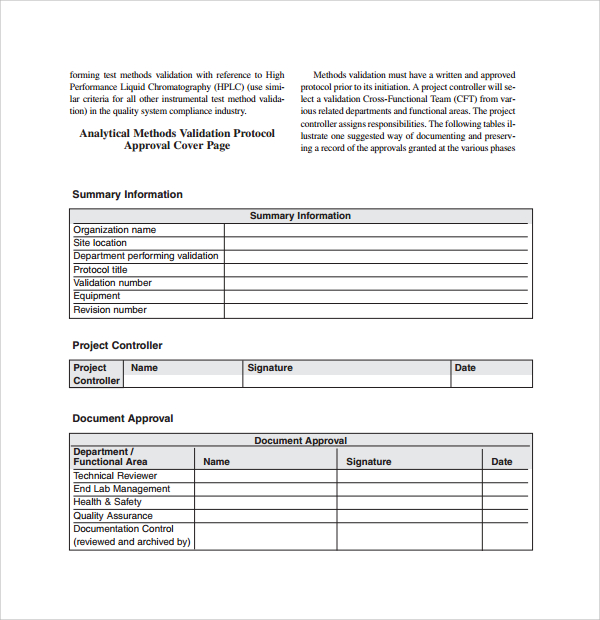
Sample Validation Plan Template 9 Free Documents In PDF Word
A Validation Master Plan or VMP is a high level document that details what how and when validation activities will be executed It also identifies the validation approach responsibilities and documentation required to ensure that quality standards and Good Manufacturing Practices GMP i e Good Clinical Practices including current Good Clinical Practices or cGCP are met

Validation Plan Template Sample By Pharmi Med Ltd Issuu
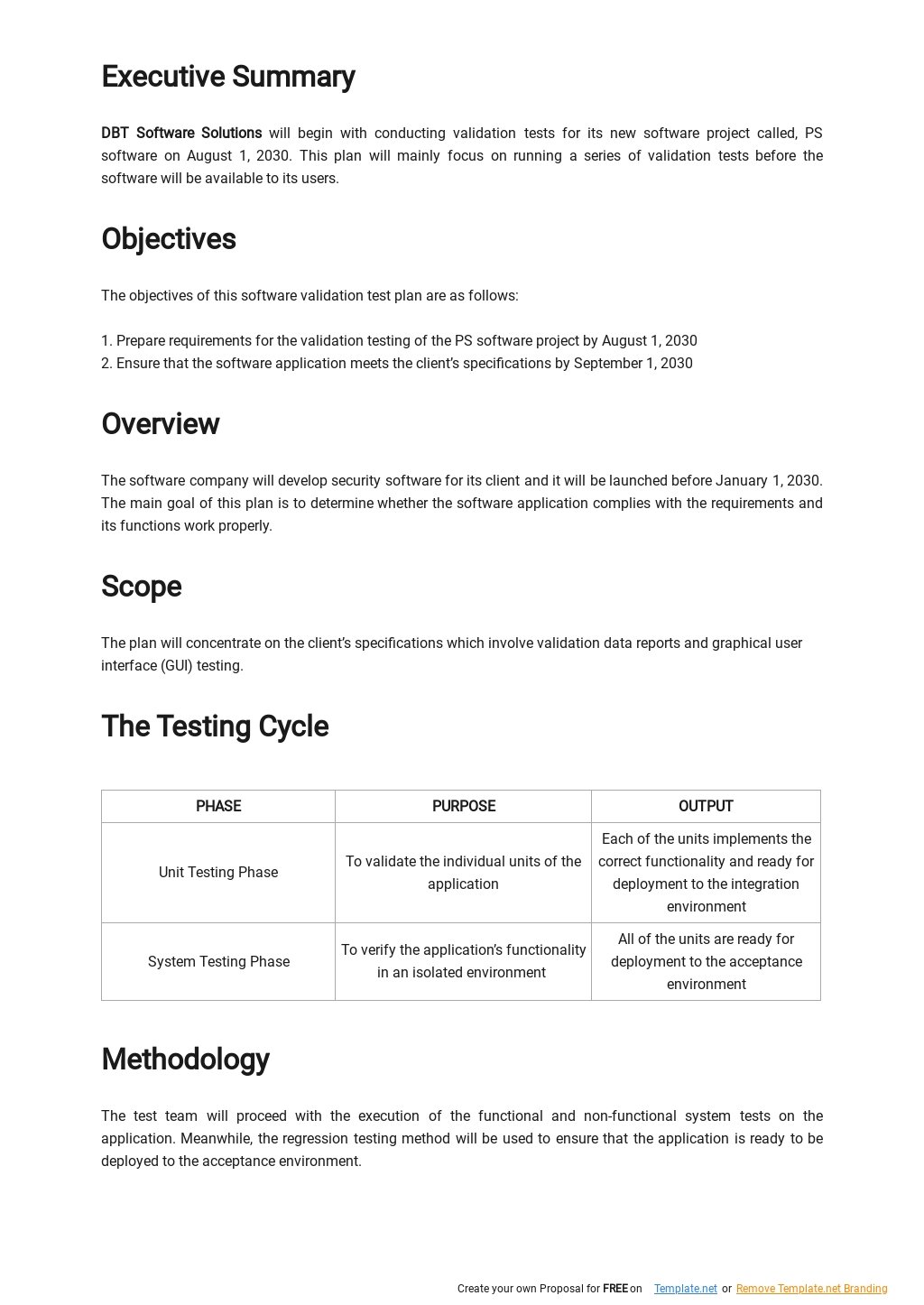
Software Validation Protocol Template
How To Write An Effective Validation Master Plan PHARMACEUTICAL ONLINE
The validation plan is the document that contains the highest level planning for the validation of a system Typically when we think of validation we think of testing however the overall validation plan must encompass a broader scope

Validation Plan Template Validation Center
Loosely to maintaining appropriate validation data How ever the practice of validation is implied more strongly in 211 68 a Automatic mechanical or electronic equipment or other types of equipment including computers or related Table A Validation program Validation Program Validation Master Plan VMP Documents Intent and
Templates. Includes templates that can be customized for Qualitative Validation Plans and Qualitative Validation Summaries. Validation Plan for Qualitative Method. Customizable template for creating a validation plan for a Qualitative method. Validation Summary Report for Qualitative Method. Customizable template for creating a validation ...
Span Class Result Type
The purpose of the record is to develop a plan for validation and verification activities in the design and development process The document is optimized for small and medium sized organizations we believe that overly complex and lengthy documents are just overkill for you This document is an appendix to the main document which is sold

Master Validation Plan Template

Software Validation Plan Template Awesome Validation Plan Template
Endata Validation Plan Template
Loosely to maintaining appropriate validation data How ever the practice of validation is implied more strongly in 211 68 a Automatic mechanical or electronic equipment or other types of equipment including computers or related Table A Validation program Validation Program Validation Master Plan VMP Documents Intent and
It is a document that describes the overall strategy for validating a process or system Organizations use it to ensure that their processes and systems comply with quality safety and regulatory standards It outlines the validation activities objectives scope approach and responsibilities

Free Validation Templates

Verification And Validation Plan Template Software Development

Validation Master Plan Understand The Importance And Benefits

All About Clinical Trial Data Management Smartsheet
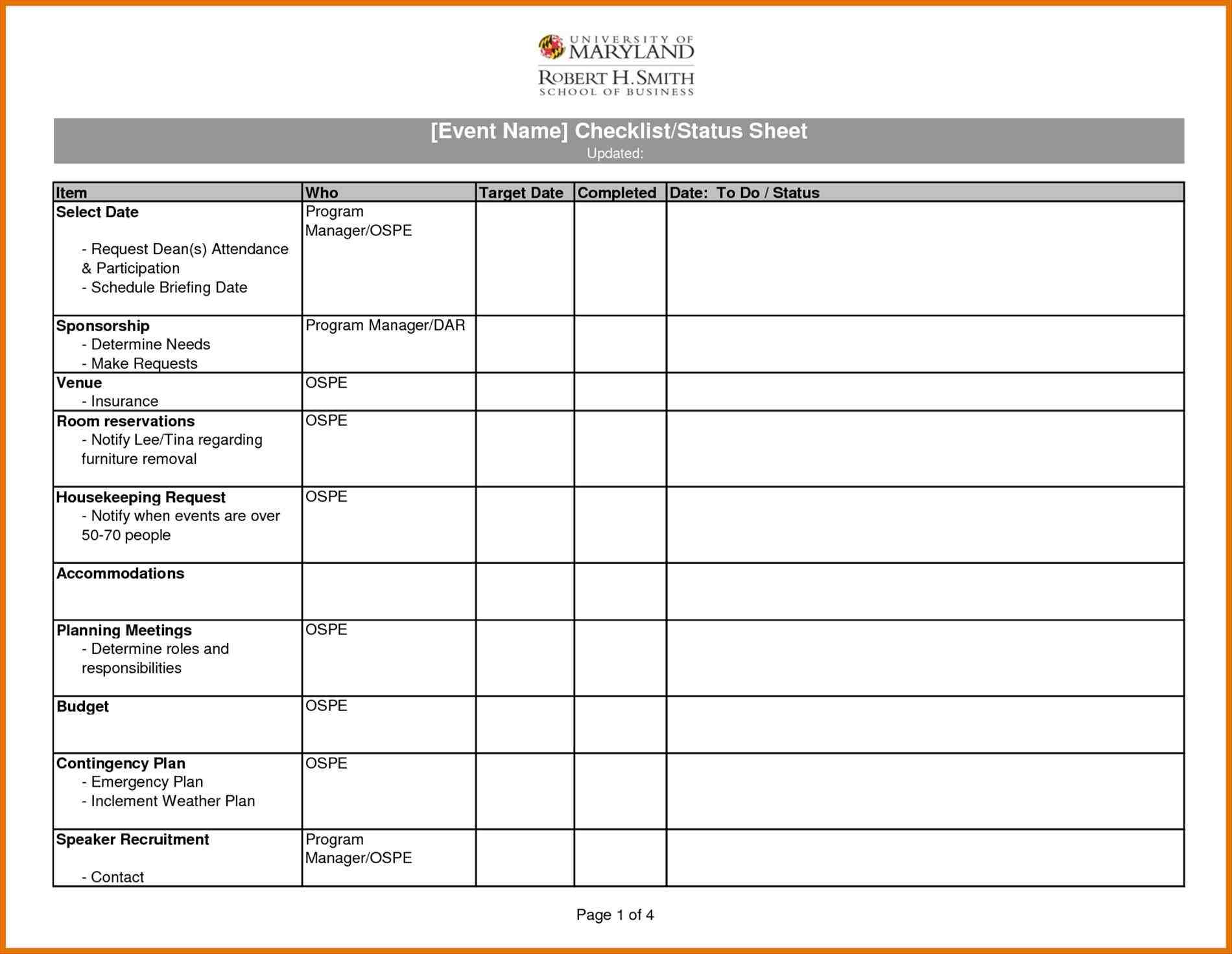
Spreadsheet Validation Template Google Spreadshee Excel Spreadsheet