Enfda 1572 Template - On 20 May 2021 the FDA released a draft information sheet guidance for sponsors clinical investigators and institutional review boards IRBs entitled Frequently Asked Questions Statement of Investigator Form FDA 1572 Revision 1 The guidance draft proposes to revise responses to the following frequently asked questions from the
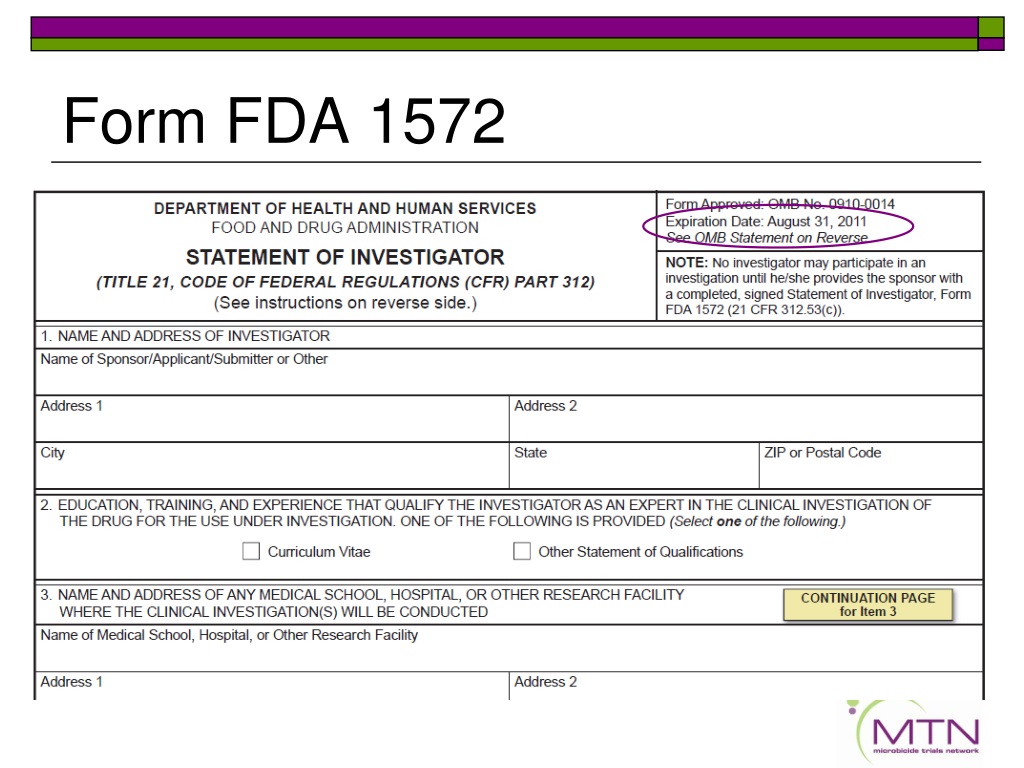
STATEMENT OF INVESTIGATOR TITLE 21 CODE OF FEDERAL REGULATIONS CFR PART 312 See instructions on reverse side NOTE No investigator may participate in an investigation until he she provides the sponsor with a completed signed Statement of Investigator Form FDA 1572 21 CFR 312 53 c NOTE No investigator may participate in an
Enfda 1572 Template

Enfda 1572 Template
Office of Sponsor and Regulatory Oversight Document #: FI01 -406 S01 Form FDA 1572 Instructions Revision #: 4 Effective Date: 15APR2021 Page 1 of 6 . General Information and Instructions
Provide a brief clinical history of the patient including 3 Indicate the proposed treatment plan including 4 Include the chemistry manufacturing and controls information and pharmacology and
Span Class Result Type
Download the Statement of Investigator Form FDA 1572 which is required for clinical trials involving investigational new drugs Learn how to fill out the form what information to provide and

Free Transport Letterhead Template Illustrator InDesign Word Apple
The Form FDA 1572 DAIDS IoR Form must list all IRBs ECs REs Approving Entity ies that are responsible for the review and approval of a clinical trial at a CRS prior to the CRSs initiation of the protocol

Id Card Template Brochure Template Corporate Id Real Id

Funeral Invitation Template Choco 1572 Doc Formats Invitation
FDA Releases Draft Guidance About Form FDA 1572 CITI Program
This guidance applies to clinical investigations conducted under 21 CFR Part 312 Investigational New Drug Applications or IND regulations It describes how to complete the Statement of
PPT INVESTIGATOR RESPONSIBILITIES PowerPoint Presentation Free
For studies approved by the UH Case Medical Center IRB following is the name and address IRB Administration Office University Hospitals Lakeside 1400 11100 Euclid Avenue Cleveland OH 44106 MS LKS 7061 216 844 1529
Financial Disclosure Forms. Effective July 1, 2014, any investigator (including sub-investigators) listed on the Form FDA 1572 must complete a financial disclosure form/statement for all DAIDS -sponsored and/or -supported studies where DAIDS holds the IND. DAIDS Policy: Process for Collection of Financial Disclosure pdf.
Span Class Result Type
Start Preamble AGENCY Food and Drug Administration HHS ACTION Notice of availability SUMMARY The Food and Drug Administration FDA Agency or we is announcing the availability of a draft information sheet guidance for sponsors clinical investigators and institutional review boards IRBs entitled Frequently Asked Questions Statement of Investigator Form FDA 1572 Revision 1

Fda 1572 Template

Fda 1572 Template
Enfda 1572 Template
For studies approved by the UH Case Medical Center IRB following is the name and address IRB Administration Office University Hospitals Lakeside 1400 11100 Euclid Avenue Cleveland OH 44106 MS LKS 7061 216 844 1529
STATEMENT OF INVESTIGATOR TITLE 21 CODE OF FEDERAL REGULATIONS CFR PART 312 See instructions on reverse side NOTE No investigator may participate in an investigation until he she provides the sponsor with a completed signed Statement of Investigator Form FDA 1572 21 CFR 312 53 c NOTE No investigator may participate in an
FDA 1572 Institutional Review Board Health Sciences

Free Download Last Will And Testament Template Resume Examples
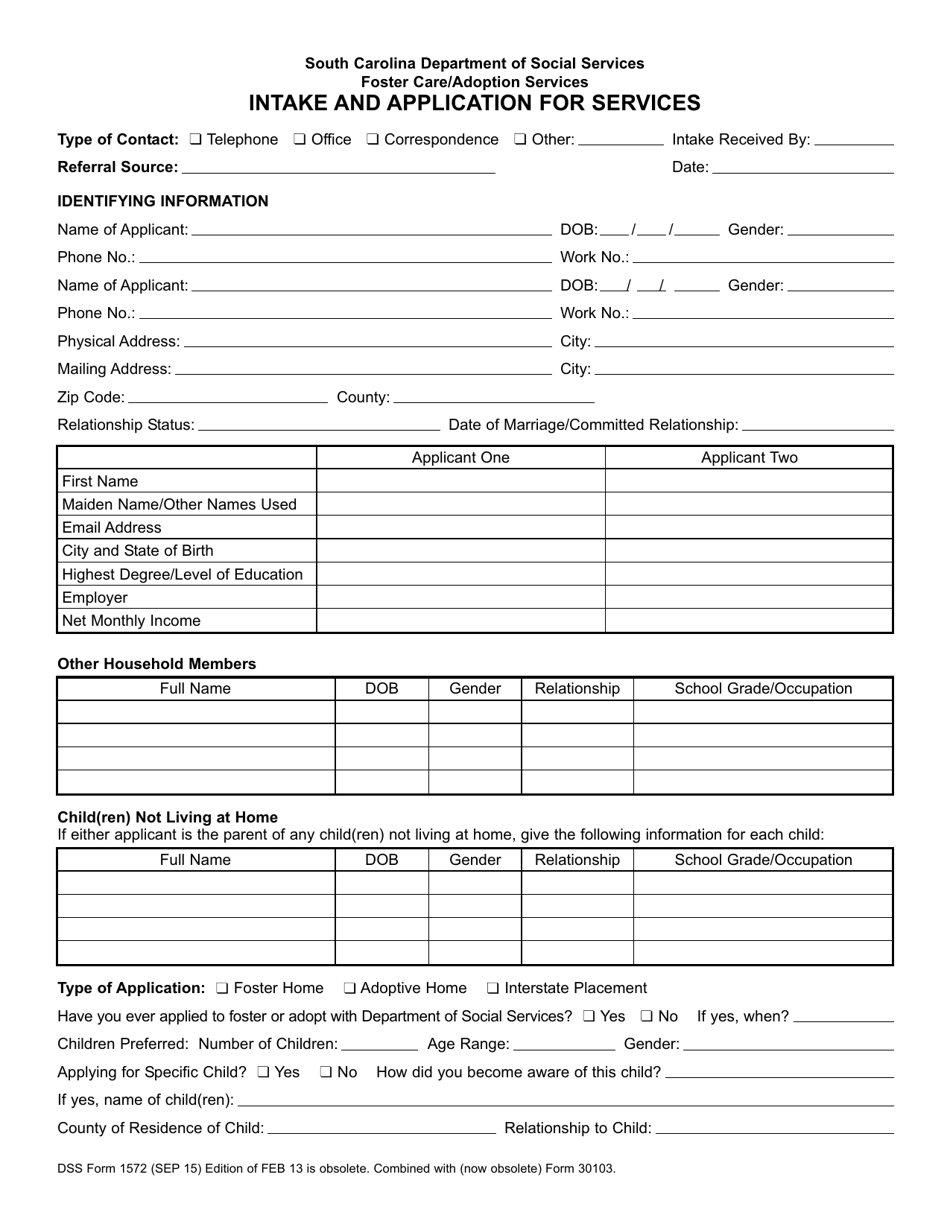
DSS Form 1572 Fill Out Sign Online And Download Printable PDF South
Leaf Stencil Clip Art Library

Top Best Free Joomla Law Firm Website Design Templates LTHEME
