Enfda Diversity Plan Template - Clinical Trial Diversity Clinical trials are research studies involving human volunteers to evaluate medical products like medications vaccines or devices for safety and effectiveness Ensuring
The United States Food and Drug Administration FDA issued draft guidance on April 13 2022 entitled Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance The purpose of the Guidance is to outline requirements for manufacturers of FDA regulated medical products to develop a Race and Ethnicity Diversity
Enfda Diversity Plan Template

Enfda Diversity Plan Template
Among other initiatives, the US Food and Drug Administration (US FDA) issued a draft guidance, "Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials" on April 13, 2022, herein referred to as the Diversity Plan [ 6 ]. This draft guidance provides a framework for a ...
Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry DRAFT GUIDANCE This guidance document is being
FDA Guidance On Diversity Plans In Clinical Trials What You Need To
Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only

Diversity Strategic Plan Template Best Of Strategic Plan 2016 2020
In April 2022 The FDA released its Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trial Guidance for Industry The 12 page
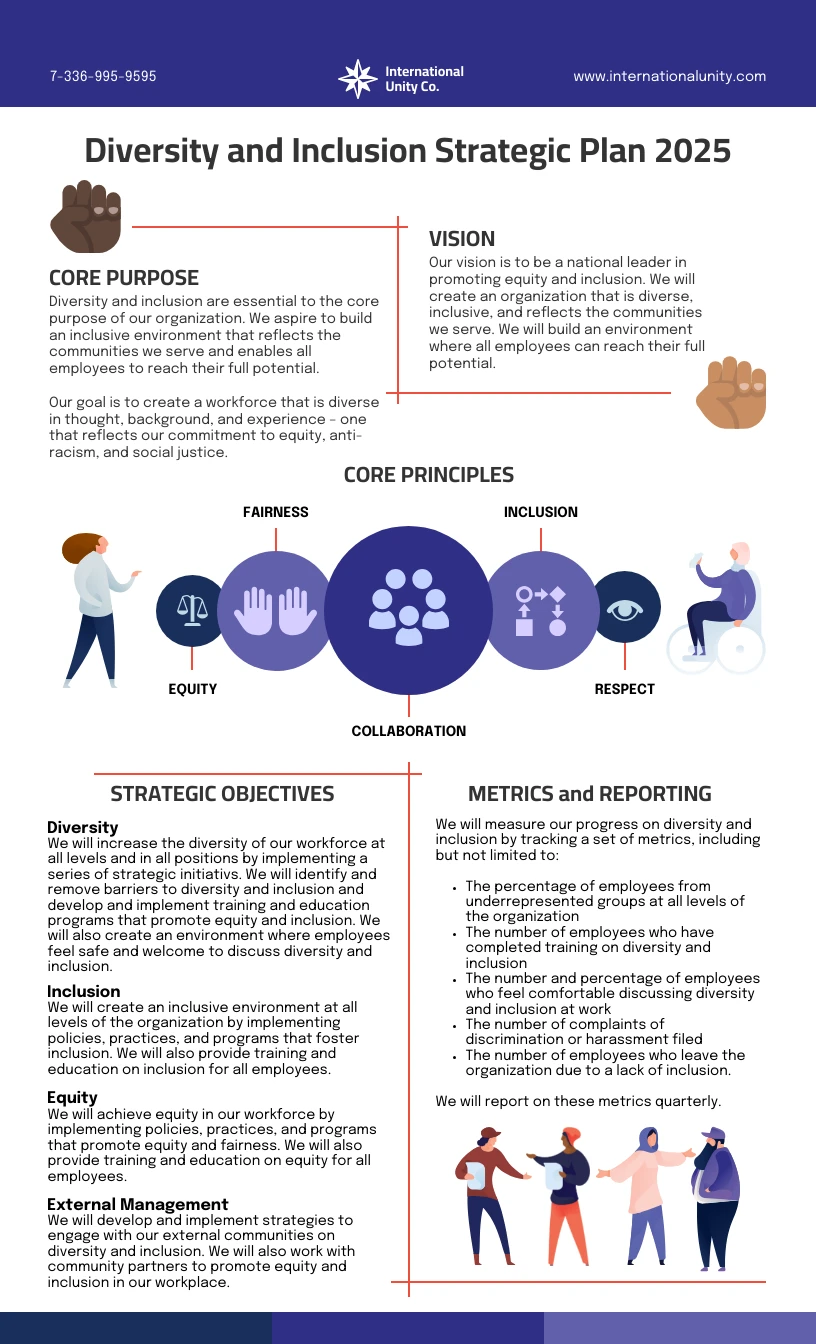
Diversity Plan Template Venngage

Diversity And Inclusion Strategic Plan Template
Clinical Trial Diversity FDA U S Food And Drug Administration
Tucked into this legislation SEC 3601 DIVERSITY ACTION PLANS FOR CLINICAL STUDIES amends the Federal Food Drug and Cosmetic Act FD C Act by requiring diversity action plans for specific FDA regulated clinical investigations

UDP Diversity Plan Input Welcome Urban Design Planning
Most notably in April 2022 the US Food and Drug Administration FDA released their draft guidance on Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials
In this whitepaper, Science 37 Diversity and Inclusion leaders share tips and strategies on how to achieve diversity in clinical trials. Continuously monitor for patient experience—while improving and adapting. And how leveraging decentralized clinical trials can improve patient access and reach. Fill out the form now to access these insights.
FDA Draft Guidance To Improve Clinical Trial Diversity Opportunities
Sponsors should update the diversity plan to provide the status in meeting enrollment goals Content of the Plan The guidance makes a number of recommendations for elements that must be included in the plan and provides a template for how the information in the plan can be organized The expected elements include
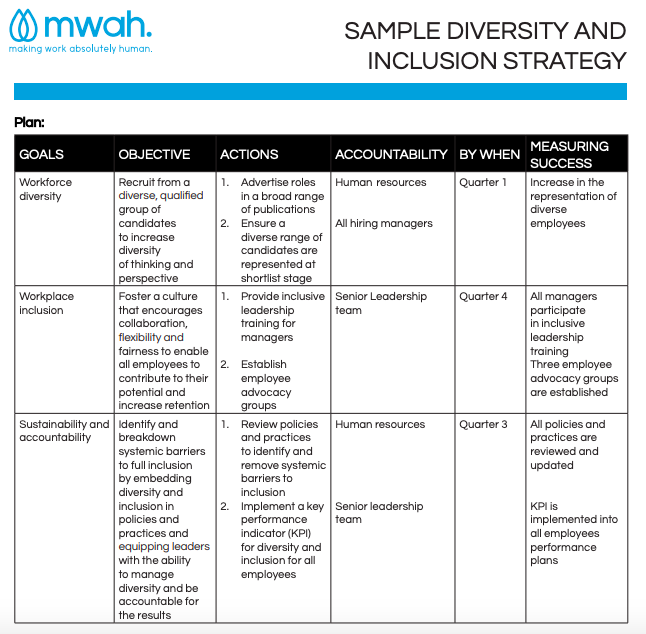
Diversity And Inclusion Action Plan Template

Fda Diversity Plan Template
Enfda Diversity Plan Template
Most notably in April 2022 the US Food and Drug Administration FDA released their draft guidance on Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials
The United States Food and Drug Administration FDA issued draft guidance on April 13 2022 entitled Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance The purpose of the Guidance is to outline requirements for manufacturers of FDA regulated medical products to develop a Race and Ethnicity Diversity

30 Diversity Strategic Plan Template Hamiltonplastering
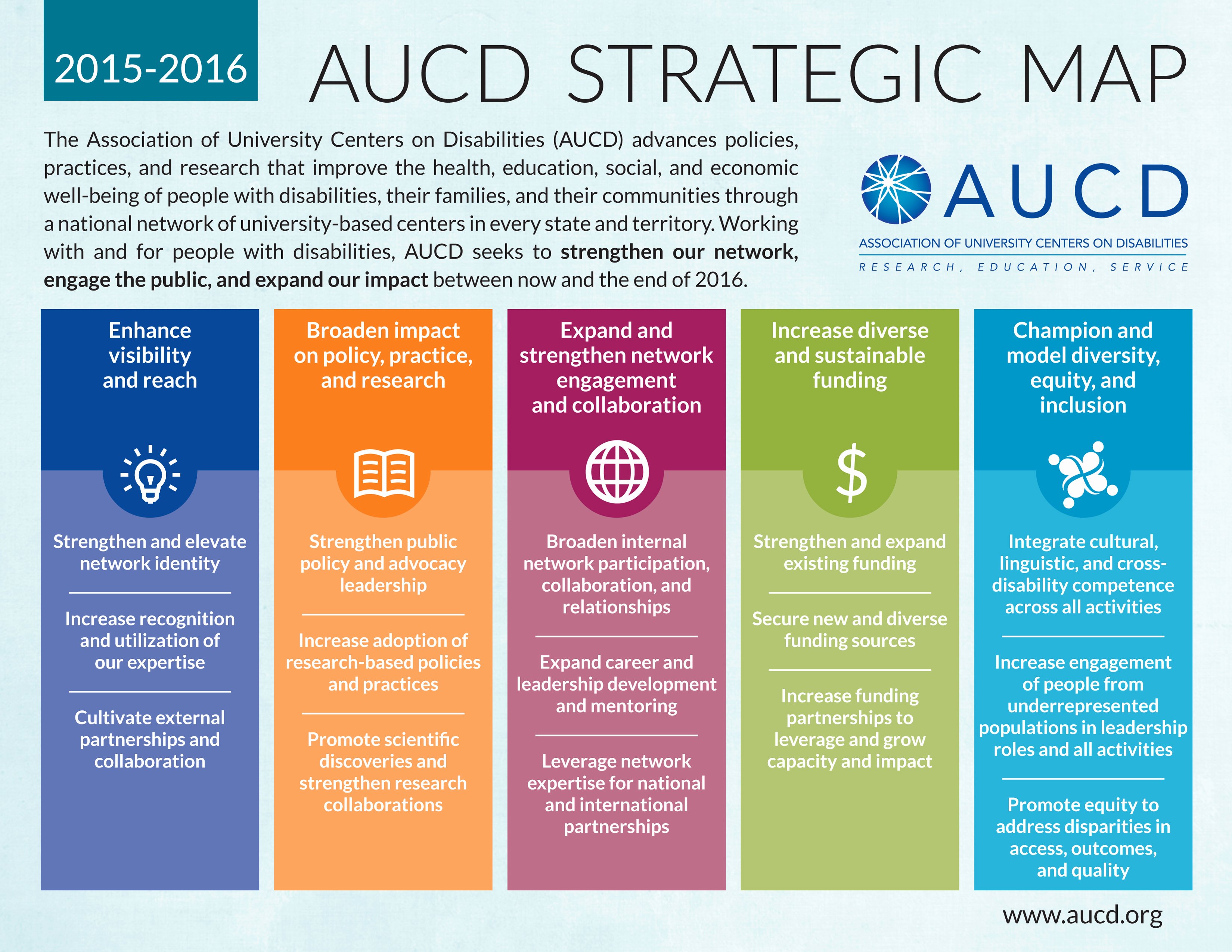
30 Diversity And Inclusion Plan Template Hamiltonplastering
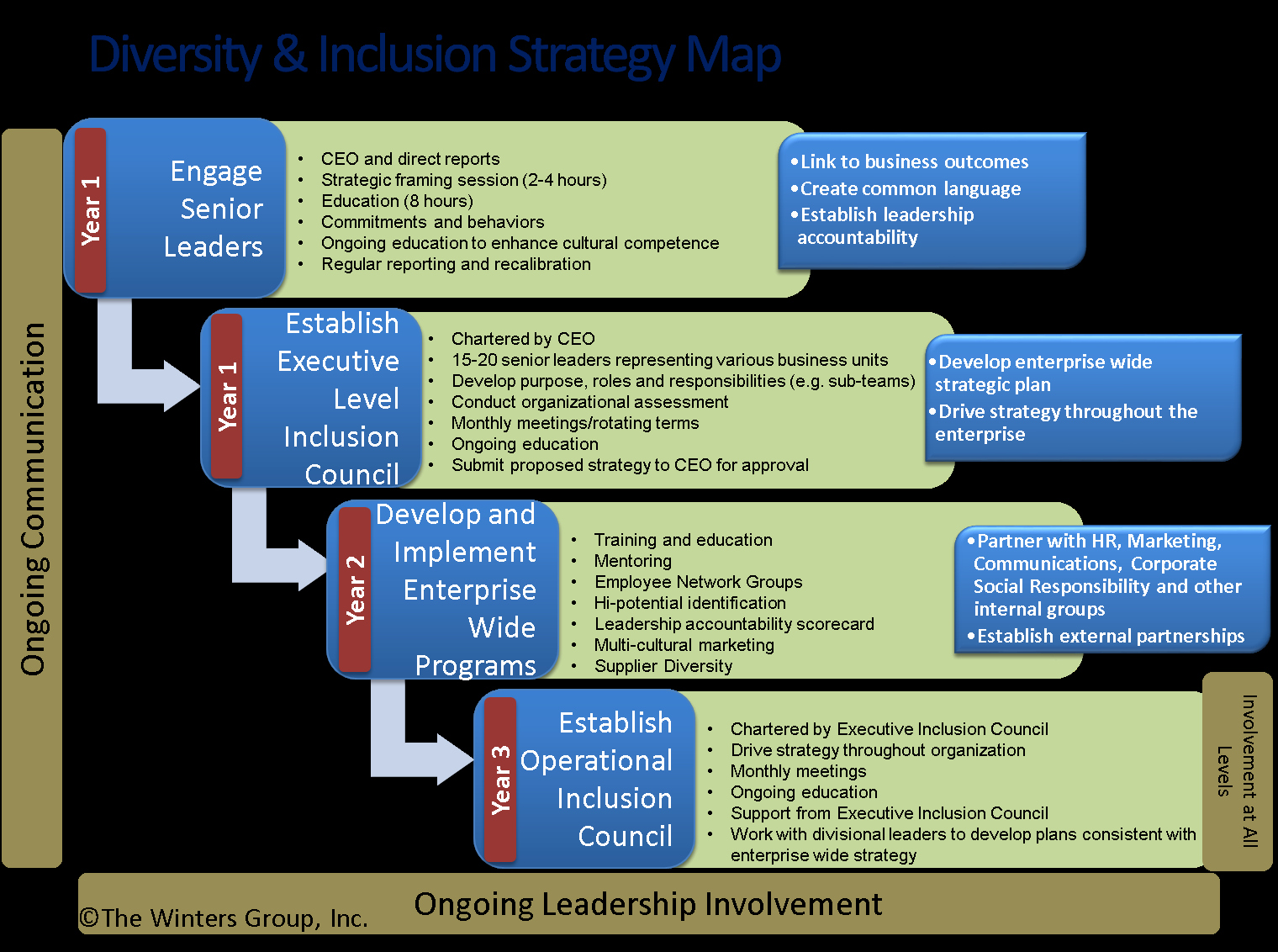
30 Diversity Strategic Plan Template Hamiltonplastering

Inclusion Diversity Framework Google Search Diversity Frameworks
Diversity Plan Diversity Business Health Care
