Eniec 62304 Software Development Plan Template - IEC 62304 is a standard for medical device software lifecycle processes The Visure Requirements ALM platform provides features and capabilities to support the various stages of the software development lifecycle including requirements management risk management traceability testing workflows change management and configuration management
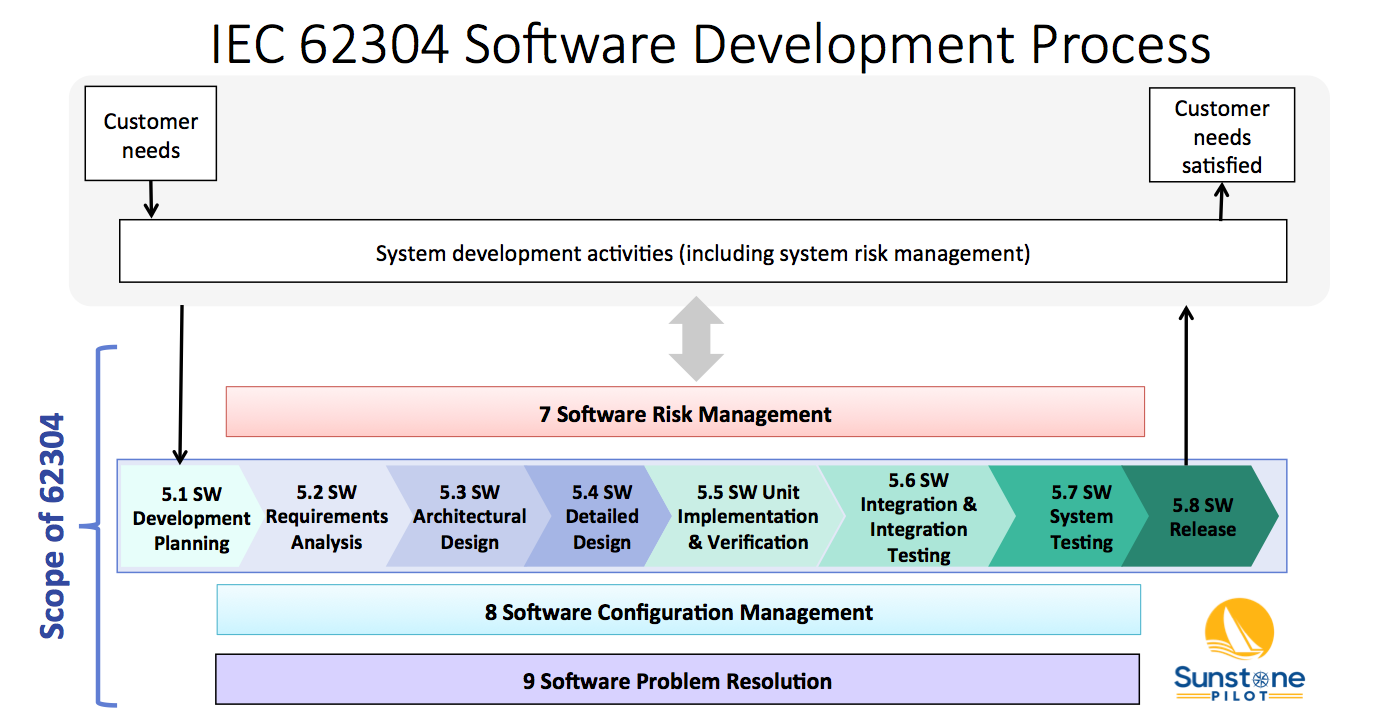
IEC 62304 EN 62304 at a Glance The IEC 62304 is a process standard it defines requirements to the development but not the product itself Evidence of the correct application of the standard i e performing the required activities is the documentation Does not want to force a development model process e g Waterfall V model
Eniec 62304 Software Development Plan Template

Eniec 62304 Software Development Plan Template
At the minimum, you need a compiler or a runtime (e.g. CPython 3.8). You'll probably also have some sort of IDE software. The IEC 62304 also specifically talks about how you "plan to avoid common software defects based on your selected programming technology". Translated to human language, this means that your programming language may ...
IEC 62304 Templates Dr Oliver Eidel The IEC 62304 describes how to develop and document software for medical devices This is an overview over our free templates which we ve published for the IEC 62304 so far In other words if you use these templates you have a pretty good chance of being compliant with the IEC 62304 Enjoy
Span Class Result Type
This is a free template provided by OpenRegulatory If you are a user of Formwork our eQMS software choose QMS on the top menu and OpenRegulatory Templates on the left menu and then open the relevant folder to find this template ready to load into Formwork If for some mysterious reason you re using a different QMS Software you can

Software Development Policy Template
Session I Session title will be inserted by editors 1 2 EuroSPI 2014 2 Related Work 2 1 Medical Device Software Quality Wallace and Kuhn 5 describe how in the years 1983 to 1991 6 of the recalls registered with the
Software Development Plan Template

Software Development Plan Template Templates Forms Checklists For
Best IEC 62304 Compliance Tools Checklists Templates
Let s write a Software Development and Maintenance Plan for CrowdCovid We start writing our first regulatory document Exciting Let s go through the template
Iec 62304 Software Development Plan Template
Organizations engaged in medical device software development are required to demonstrate compliance with a set of medical device standards and regulations before the device can be marketed One such standard IEC 62304 Medical Device Software Software
1.0 Purpose. This document is intended as a job aide to assessments for conformance to ANSI/AAMI/IEC 62304 It serves as a checklist and provides space to map the internal process to the standard's requirements. The information collected can be used as a mapping of the internal process to 62304 to aide 3rd party conformance assessments.
Writing A Software Development And Maintenance Plan OpenRegulatory
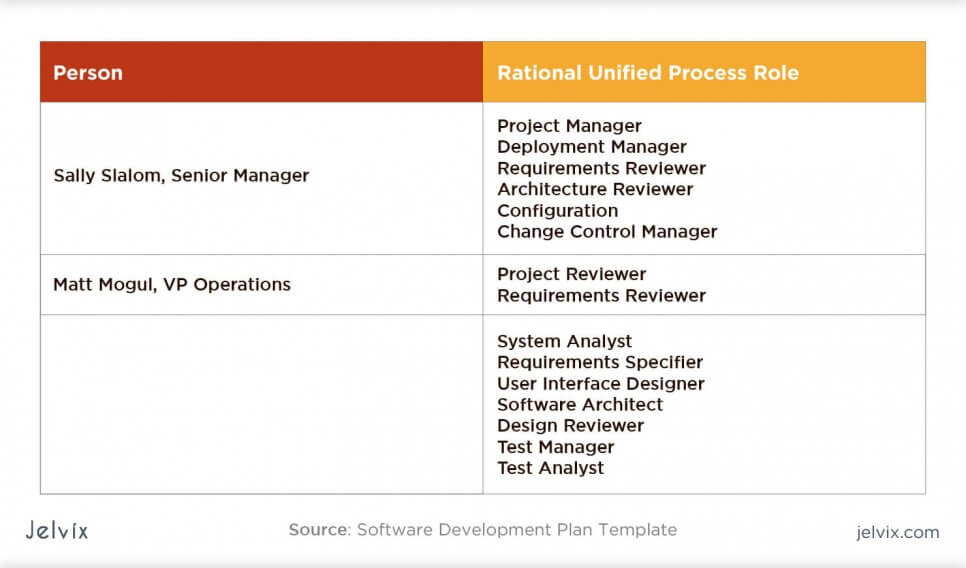
They amplify the Project Management Plan Template when it is not detailed enough to give all necessary information about the organization of a project These templates deal with sections of IEC 62304 about project organisation software configuration and problem resolution Use them to answer to those requirements of the standard
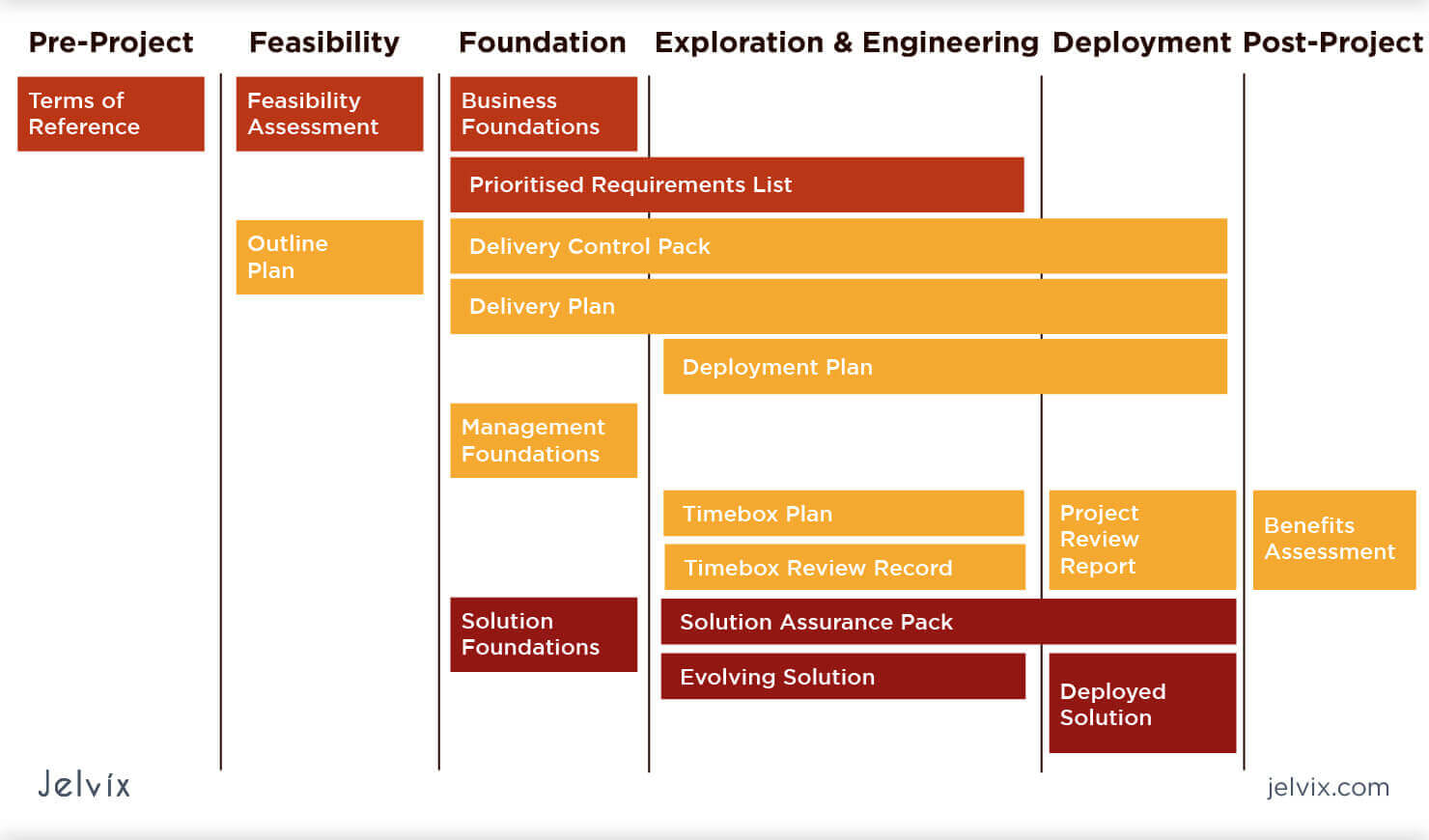
A Full Guide To Software Development Project Planning Jelvix

IEC 62304 Medical Device Software Life Cycle
Eniec 62304 Software Development Plan Template
Organizations engaged in medical device software development are required to demonstrate compliance with a set of medical device standards and regulations before the device can be marketed One such standard IEC 62304 Medical Device Software Software
IEC 62304 EN 62304 at a Glance The IEC 62304 is a process standard it defines requirements to the development but not the product itself Evidence of the correct application of the standard i e performing the required activities is the documentation Does not want to force a development model process e g Waterfall V model

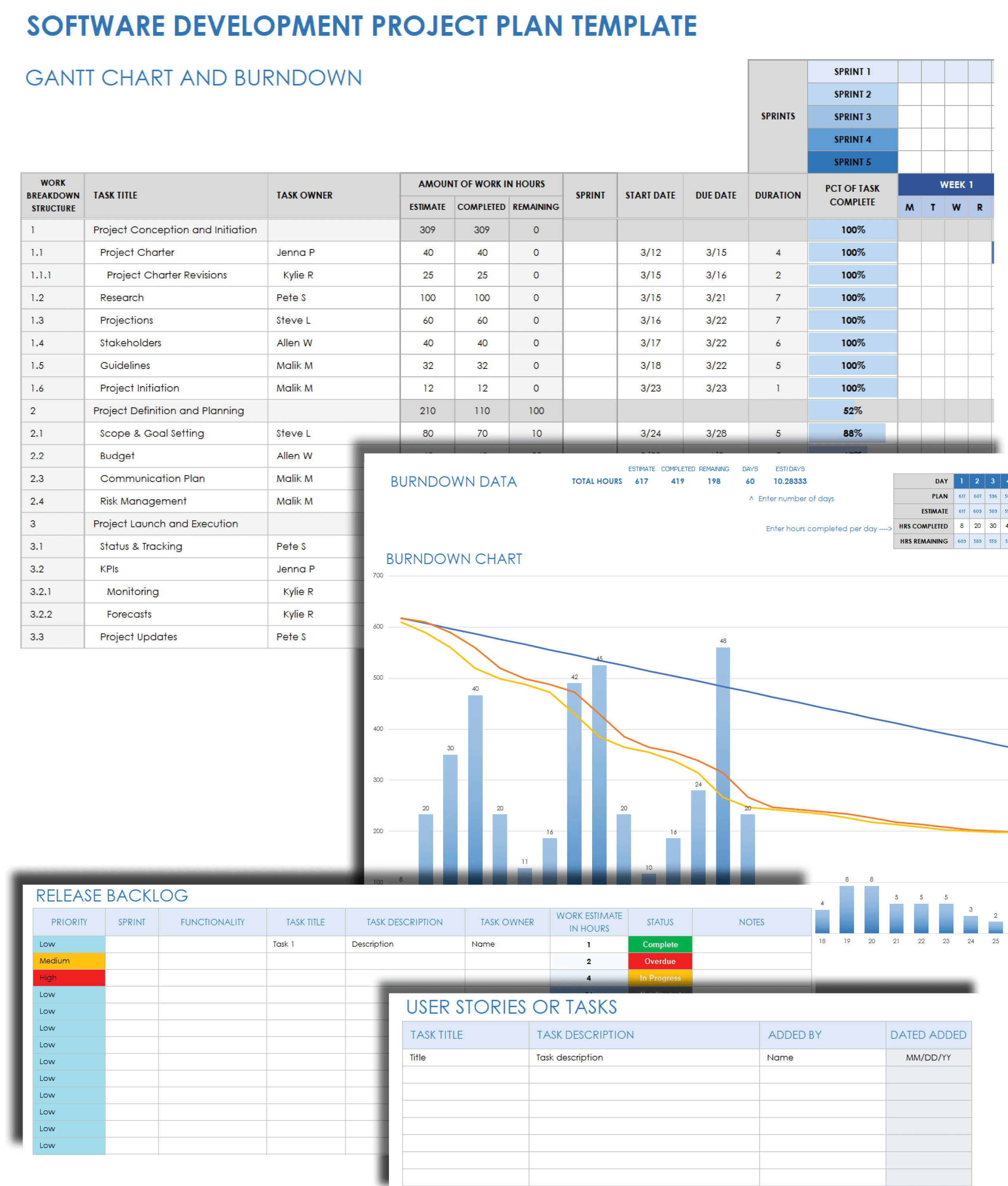
Software Development Project Plan Template Teamgantt Gambaran
Software Development Plan Template

Software Development Plan Template Templates Forms Checklists For

Software Development Proposal Template Doc Free Printable Templates
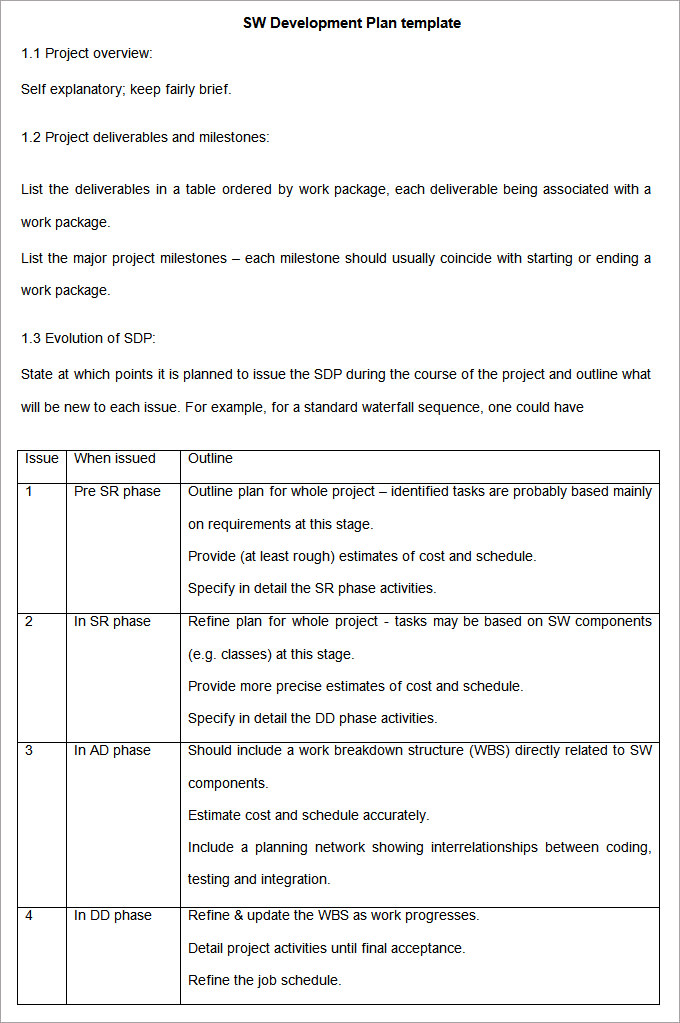
Software Development Plan Template