Enmedical Device Project Plan Template - The success of your medical device is contingent upon having a comprehensive project plan to guide its development To help access our project plan example It s tailored exclusively to medical devices so you can keep all of the details of development straight The document below shares a plan so you can see how to fill it out properly and
The project plan is what emerges from all of this preparatory work The goals of the plan are to Describe a detailed development plan for the medical device Create a realistic budget and request approval of the resources necessary to execute the project plan Assign roles and responsibilities
Enmedical Device Project Plan Template

Enmedical Device Project Plan Template
14. ». A library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them.
Legal Info A free planning resource to help you keep your medical device project on track and running smoothly
Medical Device Project Planning The Roadmap To FDA Approval
This template will provide you with a framework to complete your design and development plan It may also be used as a benchmark on your existing plan The template includes topics as required by clause 7 3 2 of ISO 13485 2016 and 820 30 b as required by 21 CFR 820 This template is instantly available for free to our newsletter subscribers
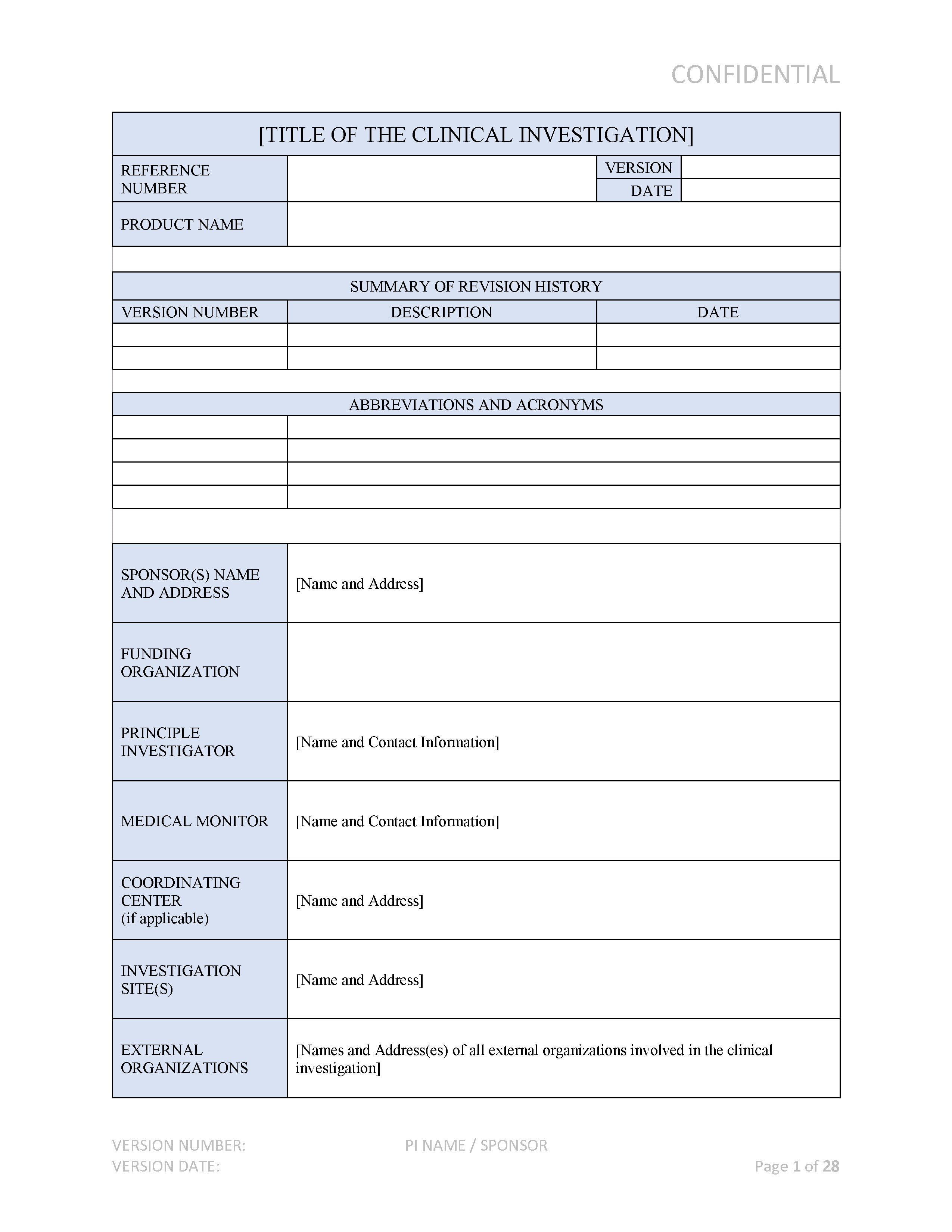
Medical Device Clinical Investigation Plan CIP ISO 14155 2020 Compliant
A project template is a specific and repeat able format for the purpose of uniformly describing processes plans and other documentation Following this template results in a reliable product with a defined development trail The results of this process are open to review by government or management agencies While the use of project templates

Basic Nursing Care Plan How To Create A Basic Nursing Care Plan

Free Site Security Management Plan Template Google Docs Word Apple
Medical Product Project Planning Guide Template
Main Elements of Medical Device Project Charter Template Custom Statuses Track the progress of your medical device projects with custom statuses tailored to your workflow such as In Progress On Hold and Completed Custom Fields Capture essential information about your projects using custom fields like Project Owner Target Launch Date
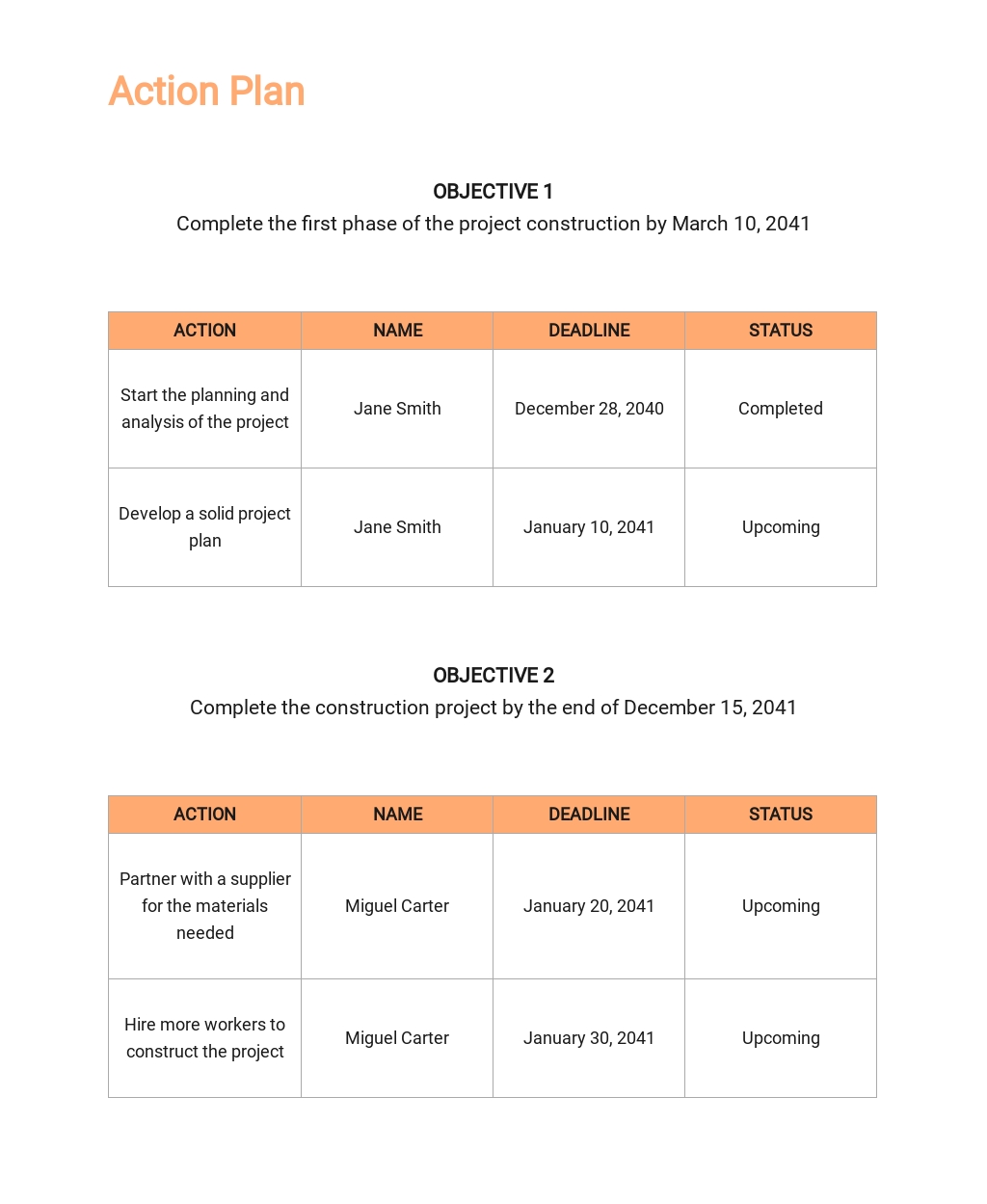
Scope Management Plan Free Template Image To U
The regulatory requirements for medical device design and development planning can be found in 7 3 2 of ISO 13485 and FDA 21 CFR Part 820 30 b 7 3 2 of ISO 13485 2016 states The organization shall plan and control the design and development of a product As appropriate design and development planning documents shall be maintained and
0 € (excl. GST/VAT) This template will provide you with a framework to complete your design and development plan. It may also be used as a benchmark for your existing plan. The template includes topics as required by clause 7.3.2 of ISO 13485:2016 and §820.30 (b) as required by 21 CFR 820. Out of stock.
Free Medical Device Templates Checklists Greenlight Guru
I encourage everyone that authors or consumes a medical device project plan to consider these points and see how they fare Here are six principles that I keep in mind for Medical Device Project Plan success 1 Listen If I m spoon feeding information I m giving the team my plan and will fail to get to our plan 2 Start high

Individual Learning Monitoring Plan Sample Ready Made Template Deped

Sample Scopes Of Work Awesome Phasing Of Construction Scope Of Work
Enmedical Device Project Plan Template
The regulatory requirements for medical device design and development planning can be found in 7 3 2 of ISO 13485 and FDA 21 CFR Part 820 30 b 7 3 2 of ISO 13485 2016 states The organization shall plan and control the design and development of a product As appropriate design and development planning documents shall be maintained and
The project plan is what emerges from all of this preparatory work The goals of the plan are to Describe a detailed development plan for the medical device Create a realistic budget and request approval of the resources necessary to execute the project plan Assign roles and responsibilities

Simple Project Scope Management Plan Template Google Docs Word

Remediation Plans Templates Documents Design Free Download

Medical Device Project Plan Free Download

Marketing Plan Template Report Template Press Release Template Law

Medical Device Project Planning The Roadmap To FDA Approval