Enmedical Device Verification And Validation Plan Template - Validation is the process of making sure that you have objective evidence that user needs and intended uses are met It is usually done by tests inspections and in some cases analysis However the target of the validation is to make sure the user needs are met in a medical device that consistently provides the intended medical benefit in
Your design validation process must include initial production units This means the medical devices used for validation have to be built in the production environment using drawings and specifications i e design outputs by production personnel Design validation must involve clinical evaluation This means that the end user s should be
Enmedical Device Verification And Validation Plan Template
Enmedical Device Verification And Validation Plan Template
Page 1 of 10 ©2020 PROCESS VERIFICATION AND VALIDATION FOR MEDICAL DEVICES USING ADDITIVE MANUFACTURING This paper is intended for educational purposes only and does not replace independent professional judgment.
Jun 20 2019 2 Verification confirms that the device meets all the requirements What do you do to demonstrate that Typically includes functional testing etc Validation confirms that the system meets the user needs and intended use What do you do to demonstrate that Often includes some kind of clinical assessment usability studies etc
Beginner S Guide To Design Verification Design Validation For Medical
Nov 23 2021 5 Guidance for Industry Process Validation General Principles and Practices U S Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research CDER Center for Biologics Evaluation and Research CBER Center for Veterinary Medicine CVM January 2011 Current Good Manufacturing

Master Plan Template
T V S D Process validation in medical devices 5 Validation planning The Global Harmonization Task Force GHTF 3 defines process validation as a term used in the medical device

Verification And Validation Plan Template Software Development
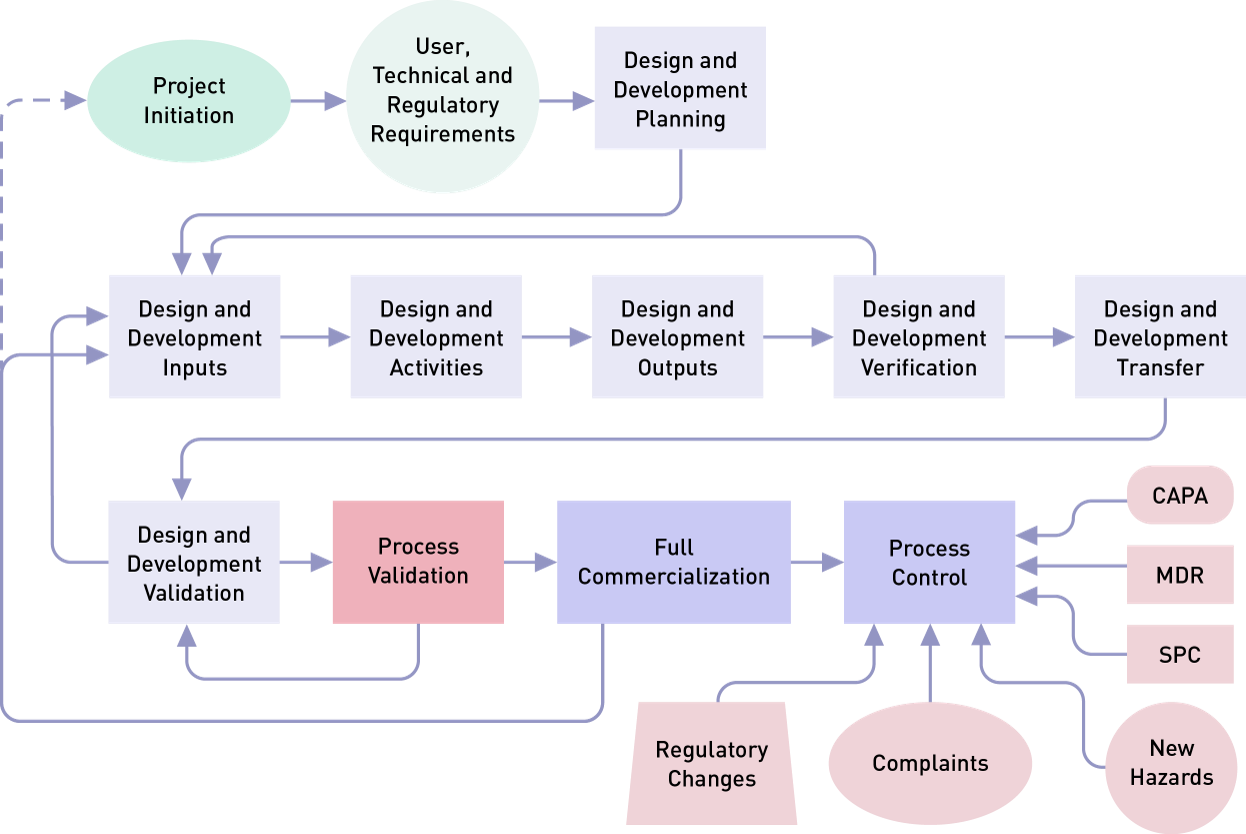
Medical Device Process Validation Overview Steps Oriel STAT A MATRIX
Validation And Verification For Medical Devices ASME
Step 1 Define the Scope and Objectives The first step in creating a Master Validation Plan MVP template for medical devices with procurement is to clearly define the scope and objectives of the plan This involves determining which aspects of procurement will be included in the validation process such as supplier qualification material
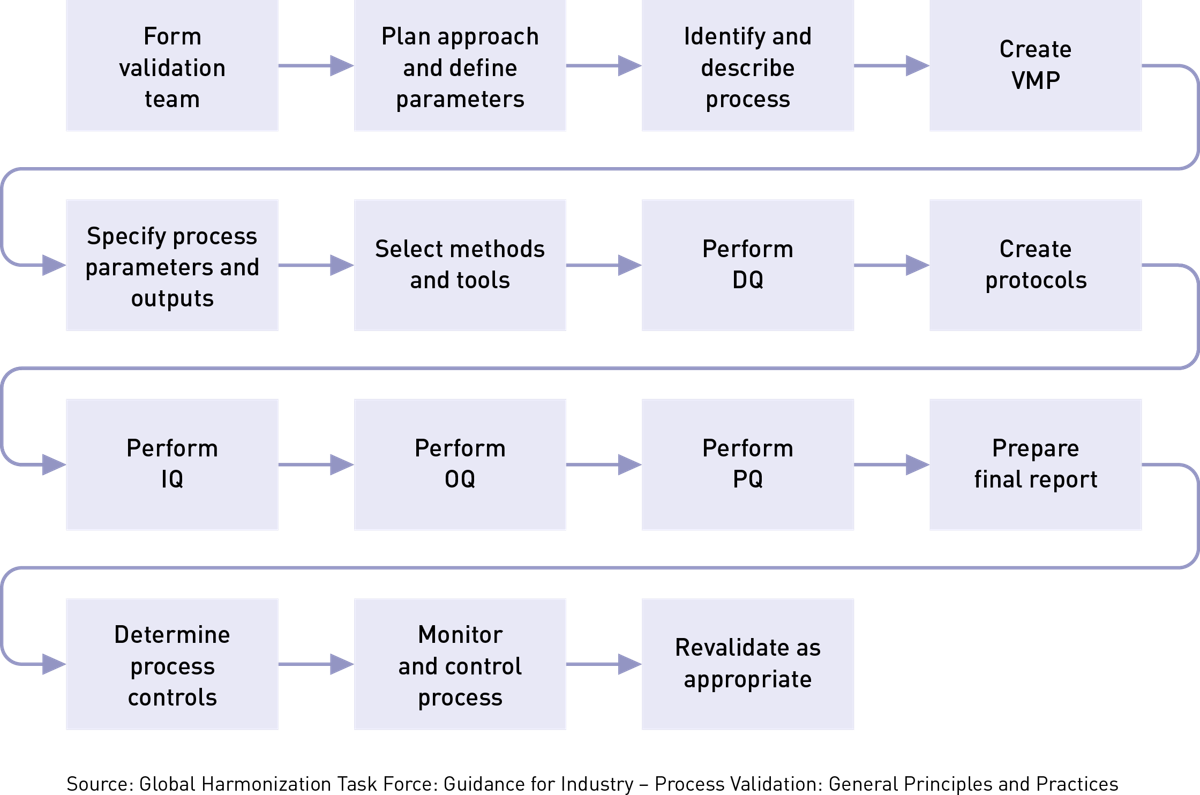
Overview Of Medical Device Process Validation IQ OQ And PQ Oriel
Design verification and design validation are essential parts of the medical device product development process Sometimes referred to as V V it s important to understand what both terms mean and how they differ if you are about to embark on a medical device product development project
IMDRF/PMD WG/N49 Definitions for Personalized Medical Devices. , establishing harmonized definitions for various categories of personalized medical devices (PMDs), including custom-made, patient-matched, and adaptable medical devices. This document introduces the concept of a specified design envelope, a characteristic feature in the definition ...
Span Class Result Type
Design validation is a testing process by which you prove validate that the device you ve built works for the end user as intended Official word from the FDA 21 CFR 820 3 states that design validation is establishing by objective evidence that device specifications conform with user needs and intended use s

Verification And Validation Model Software Development Life Cycle

Template Word Master Software Validation Test Plan According To The
Enmedical Device Verification And Validation Plan Template
Design verification and design validation are essential parts of the medical device product development process Sometimes referred to as V V it s important to understand what both terms mean and how they differ if you are about to embark on a medical device product development project
Your design validation process must include initial production units This means the medical devices used for validation have to be built in the production environment using drawings and specifications i e design outputs by production personnel Design validation must involve clinical evaluation This means that the end user s should be

Conducting Medical Device Verification And Validation Tests
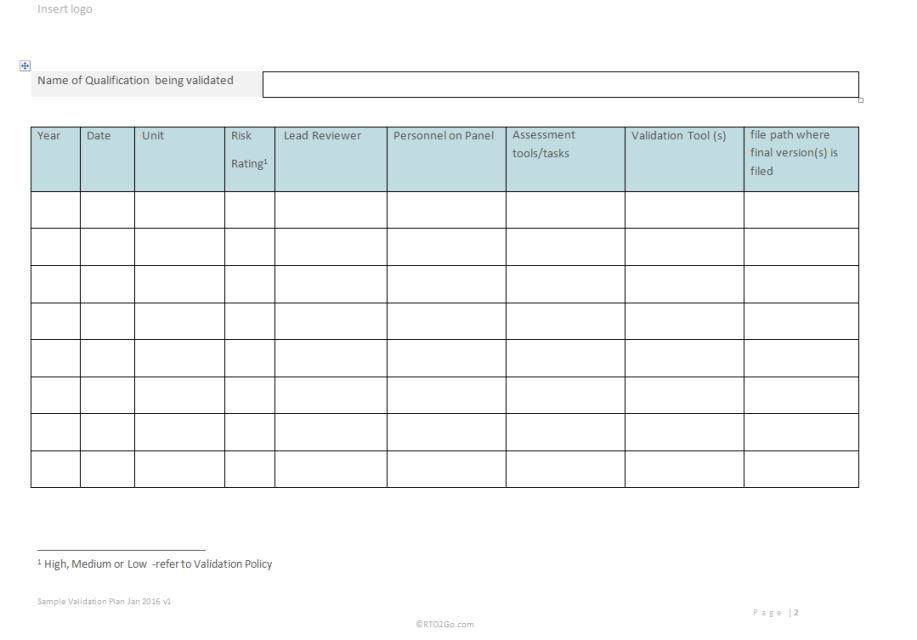
RTO2Go VET Validation Plan For RTO s

FDA Software Validation 2022 Guide Checklist Template 2022

Validation Master Plan Understand The Importance And Benefits
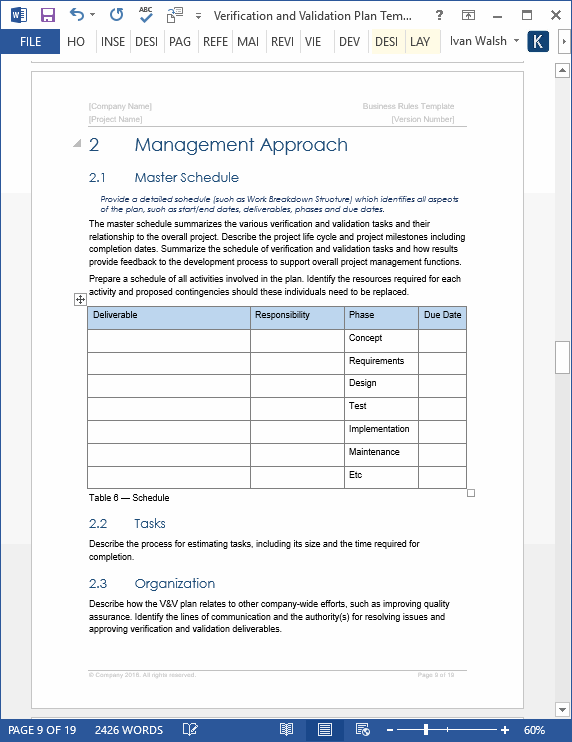
Verification And Validation Plan Template Software Development