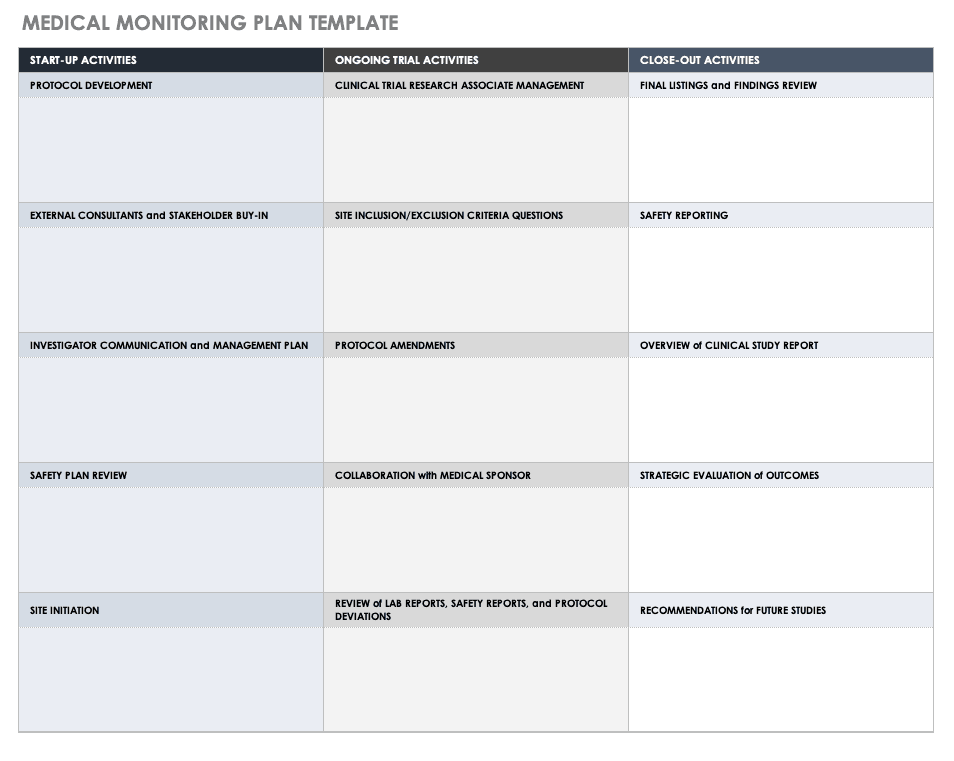
Enmedical Monitoring Plan Template - This is an MS Word template to use as a starting point for preparing a Medical Monitoring Plan for clinical trials or research Also we have included a proposed structure for a medical monitoring plan draft language and other guidance to assist you in creating a medical monitoring plan Moreover we have saved this template in different
Text enclosed with is a placeholder for a specific detail e g protocol title replace as appropriate Delete template specific instructional text as well as this Tool Summary Sheet during the monitoring plan development process Leave the template version information in the lower left hand corner of the document
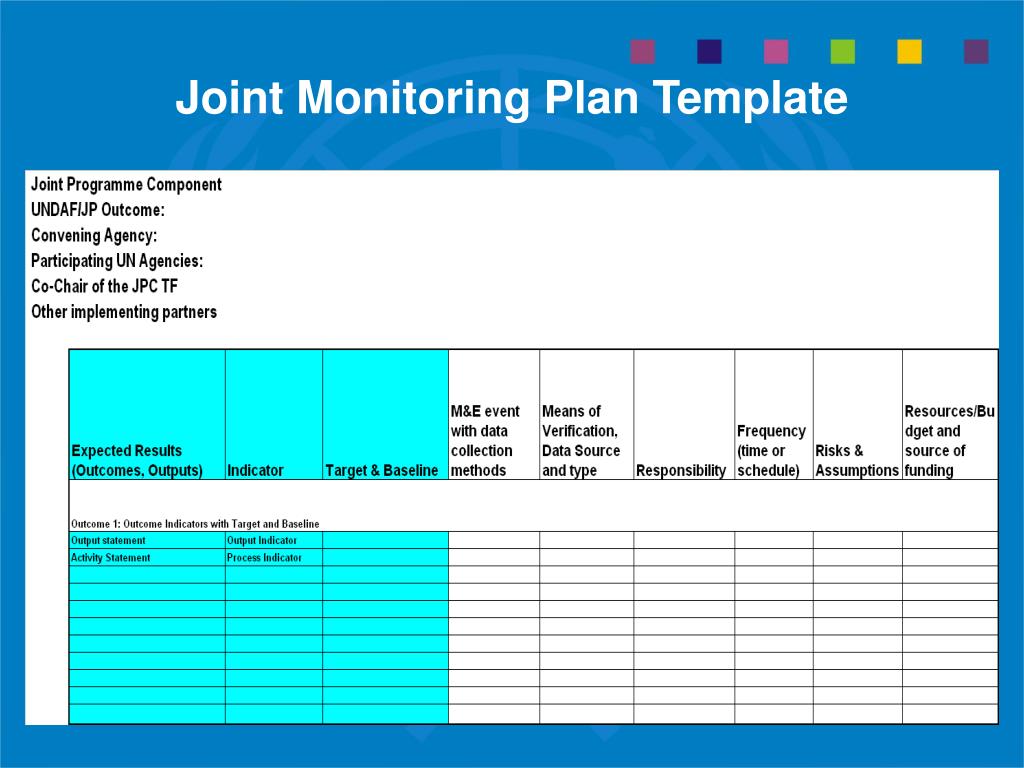
Enmedical Monitoring Plan Template

Enmedical Monitoring Plan Template
Medical Monitoring Plan 1 | P a g e o f 1 4 1.0 INTRODUCTION Medical surveillance is the systematic collection, analysis, and evaluation of health data in the
1 Good practices guidance handbook for national TB surveys How to apply good clinical and good data management practices for national TB surveys Monitoring Plan Template TOOL 1 5 INSTRUCTIONS This template is a suggested format for a Monitoring Plan developed by TB Survey Teams
Span Class Result Type
The Medical Monitoring Plan template is for healthcare professionals and teams The template helps users create an organized plan for monitoring patient medical conditions and identify the objectives action items and KPIs needed to achieve the desired results 1 Define clear examples of your focus areas
PPT Planning Monitoring Evaluation Of One Program PowerPoint
Monitoring Plan Template This ready to use template is for setting up a monitoring plan It is up to date with Swiss and international laws and recommendations and allows clinical research professionals to plan the extent of monitoring by using a risk based strategy This template is particularly useful for multicentre trials within the SCTO s CTU Network but is suitable for all sites
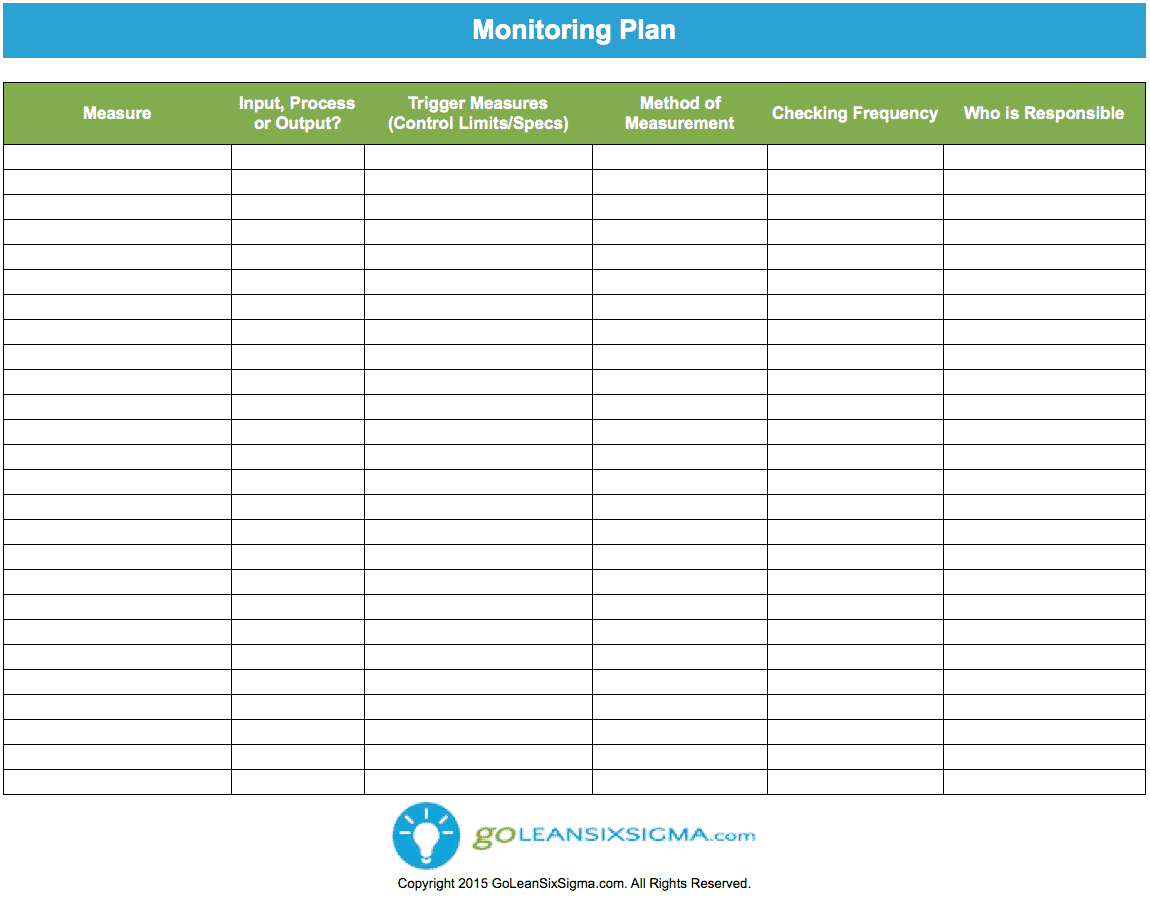
Monitoring Plan Template Example

PDF Designing A Monitoring Plan
Medical Monitoring Plan Template ICH GCP FDA EMA Easy Global Training
The first on site monitoring visit should occur within 6 8 weeks of the first particip randomisation Thereafter monitoring visits will be conducted annually until the last participant has completed the follow up evaluations according to the protocol Additional visits will only be scheduled if required
Individual Learning Monitoring Plan Template PDF
Guidance for Clinical Research Associates responsible for preparing a Clinical Monitoring Plan HHS is committed to making its websites and documents accessible to the widest possible audience including individuals with disabilities We are in the process of retroactively making some documents accessible If you need assistance accessing an
This guidance provides information on risk-based approaches to monitoring the conduct of clinical investigations2 of human drug and biological products, medical devices, and combination products.3 ...
Span Class Result Type
The goal of the DSMP is to provide a general description of a plan that you intend to implement for data and safety monitoring The DSMP should specify the following A brief description of the study design Primary and secondary outcome measures endpoints Sample size and target population
Clinical Case Review Template HQ Template Documents
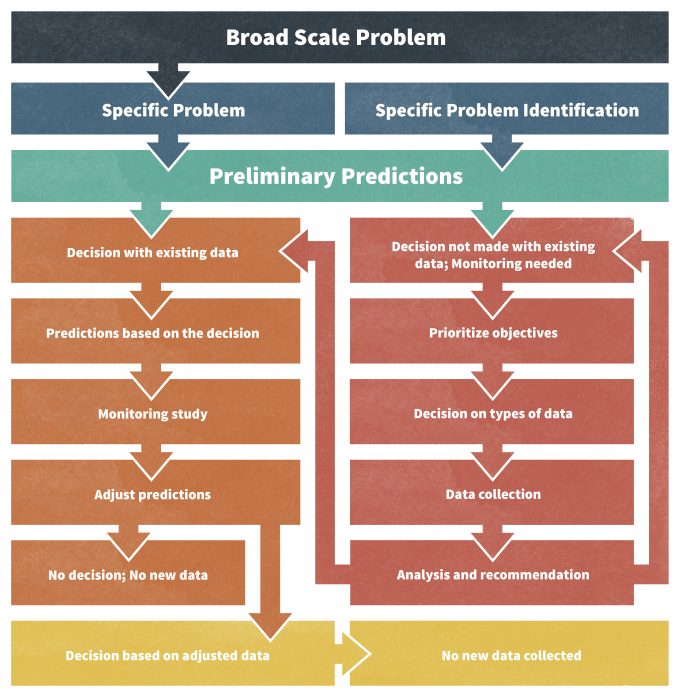
Designing A Monitoring Plan Monitoring Animal Populations And Their
Enmedical Monitoring Plan Template
Guidance for Clinical Research Associates responsible for preparing a Clinical Monitoring Plan HHS is committed to making its websites and documents accessible to the widest possible audience including individuals with disabilities We are in the process of retroactively making some documents accessible If you need assistance accessing an
Text enclosed with is a placeholder for a specific detail e g protocol title replace as appropriate Delete template specific instructional text as well as this Tool Summary Sheet during the monitoring plan development process Leave the template version information in the lower left hand corner of the document
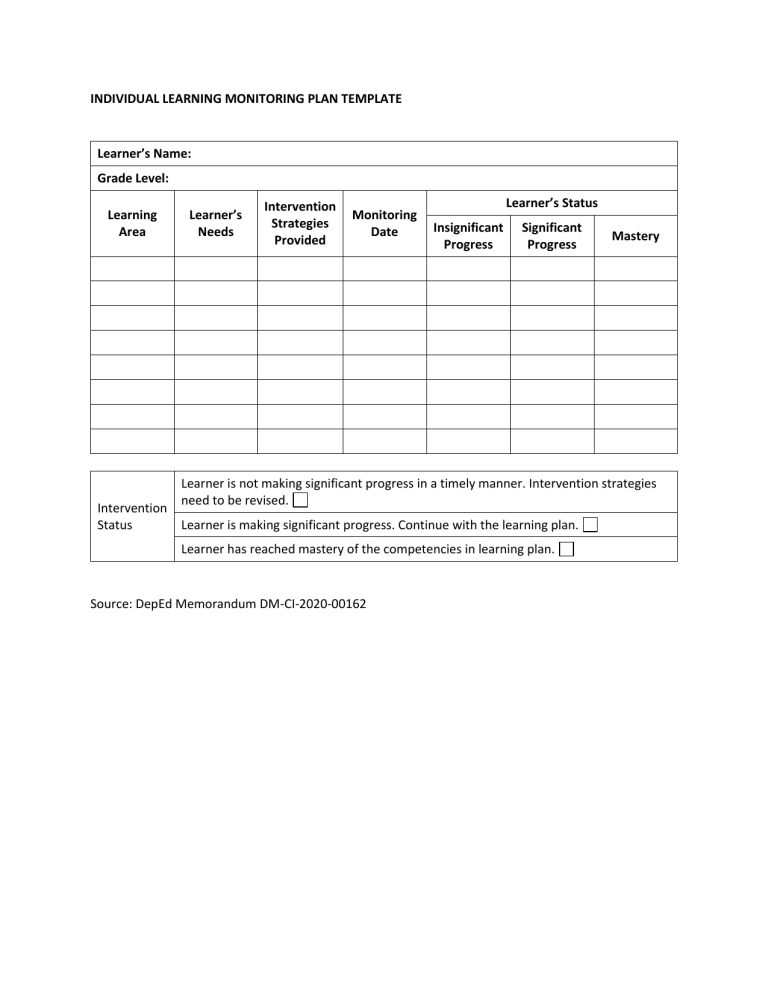
INDIVIDUAL LEARNING MONITORING PLAN ilmp

Monitoring 2 3 2 Cohesive Strategy Partnership
Site Monitoring Plan Template PDF Clinical Trial Business
Monitoring Plan PDF
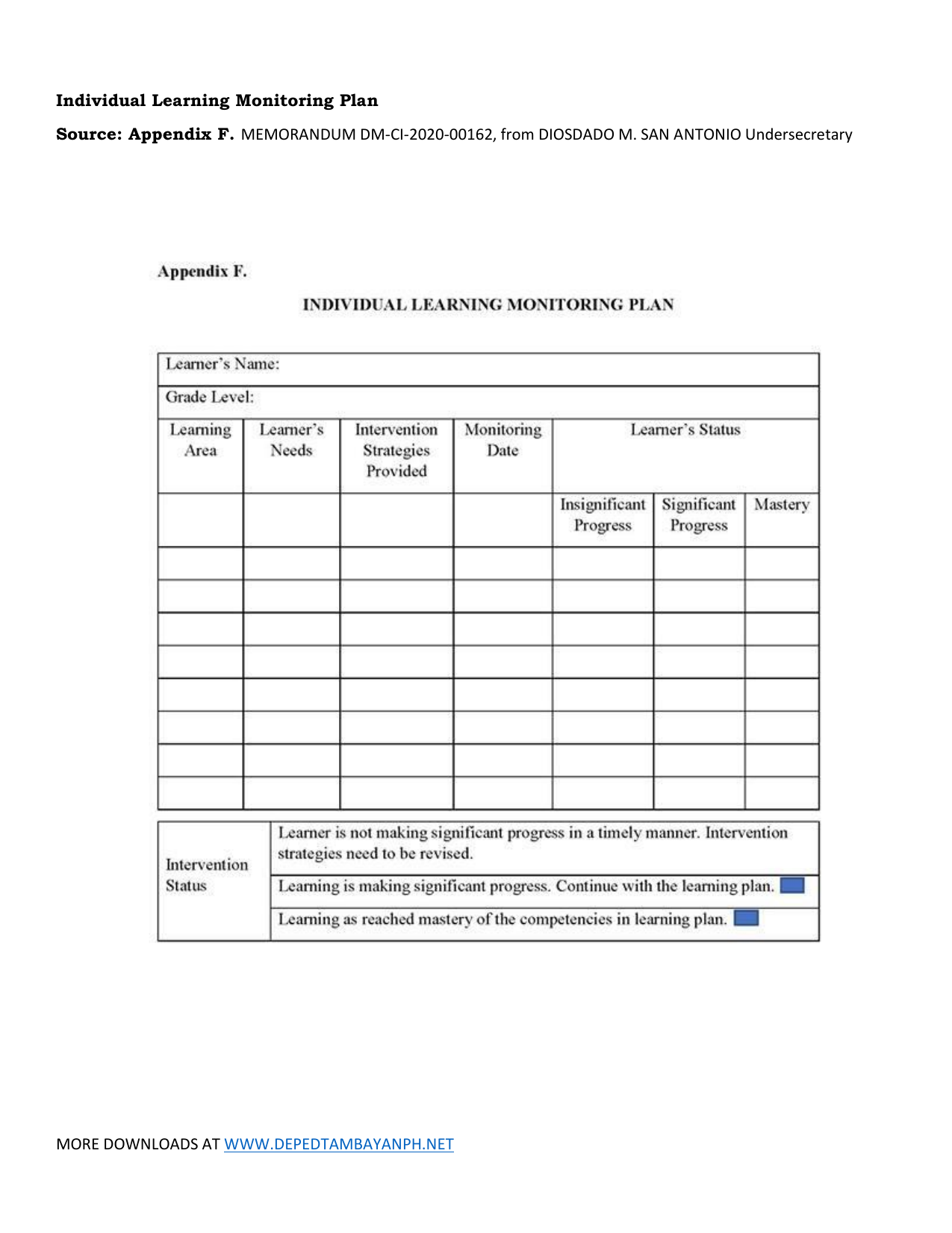
Individual Learning Monitoring Plan


