Enpharmaceutical Sop Template - Step by step pre written standard operating procedures forms templates and manuals in the area of GMP Good Manufacturing Practice GLP Production Operations Quality Assurance Management Quality Control Microbiology Laboratory Process cleaning and methodology Validation Regulatory auditing created for small and medium size pharmaceutical manufacturing environments
Calibration SOPs Production SOP QC SOP QA SOP SOP for cleaning Microbiology SOP SOP lists for all departments
Enpharmaceutical Sop Template
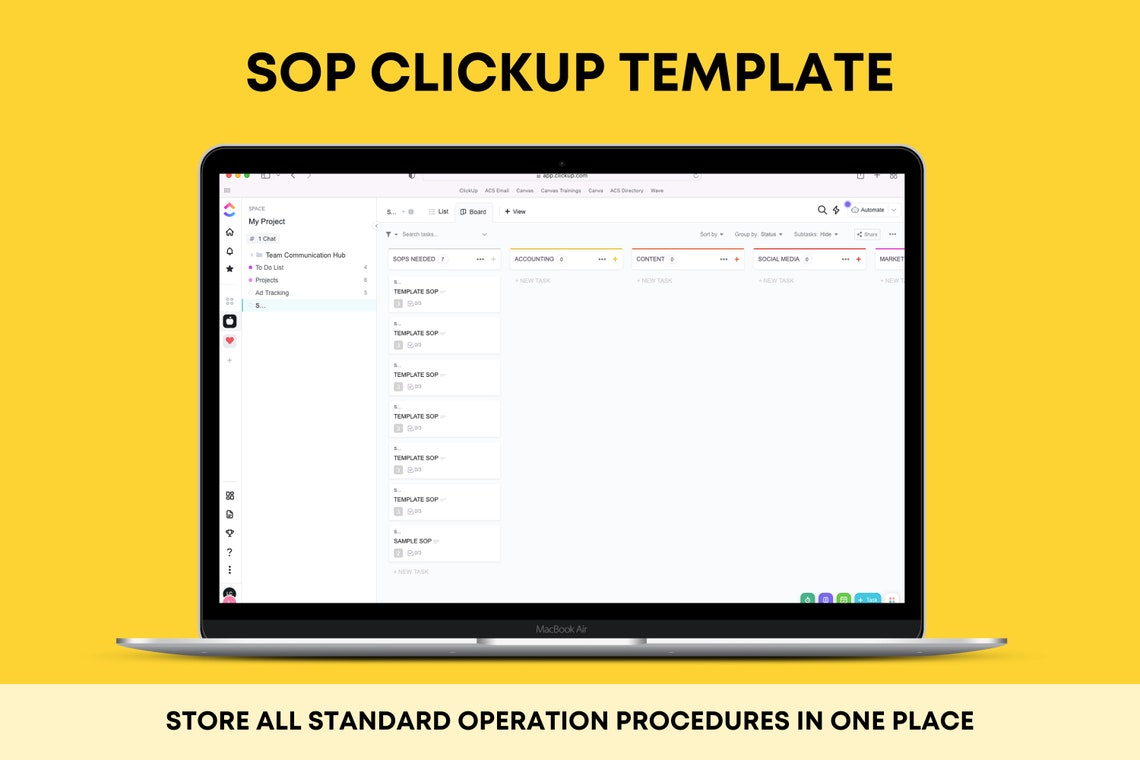
Enpharmaceutical Sop Template
Garbing Requirements. The required garb, manner of storage, and order of garbing must be determined by the facility and documented in the facility's SOPs. Category 1 and 2: The facility's SOPs must describe disinfection procedures for reusing goggles, respirators, and other reusable equipment. Category 3: The facility's SOPs must describe ...
Personnel management is the most challenging variable in maintaining current Good Manufacturing Practice cGMP across the life cycle of drug manufacture safety and supply A standard operating procedure SOP outlines agreed upon instructions for personnel training and instructions for maintaining systems machines documents and records in a qualified state to produce safe products This
Pharma SOPs Pharmaguideline
The following Model Standard Operating procedures are included in the document 1 Standard Operating Procedure for Pharmaceutical Storage Practice

SOP Template Standard Operating Procedure Template Templates
Safety SOP Specifies the steps involved in ensuring the safety of employees and drug products This includes procedures for handling hazardous materials as well as procedures for responding to accidents and emergencies Product Distribution SOP Governs the distribution and transportation of pharmaceutical products

SOP Standard Operating Procedure Lifecycle PowerPoint Template

Chemical Storage And Handling SOP Template GMPWebSource
Pharmaceuticals Quality Assurance Validation Procedure SOP
This article presents step by step instructions and expert tips on how to write standard operating procedures SOPs We provide free easy to use Word and PowerPoint SOP templates along with a checklist to prepare for and write SOPs Included on this page you will find steps on how to write a standard operating procedure detailed SOP

Standard Operating Procedure SOP Template Paper Chaser
1 Purpose This SOP defines the actions and responsibilities for the handling of reports of suspected defective centrally authorised medicinal products received by the EMA Secretariat and which may require immediate action This SOP applies to all defective product reports and to all reported product quality problems received by any EMA staff
Below is a simple standard operating procedure outline and sample sop format: Title Page - contains the complete SOP title, SOP number, date of approval or version number, the name and signature of the author, the name and signature of the person authorizing the SOP, and their dates of signing respectively.; Table of Contents - indicate page numbers and the total number of pages of the SOP
Span Class Result Type
List other SOPs which directly affect or are relevant to this procedure For example the SOP for making a buffer used in the procedure or the SOP for the operation of a piece of equipment used in the procedure

SOP Template Standard Operating Procedure Human Resources Etsy
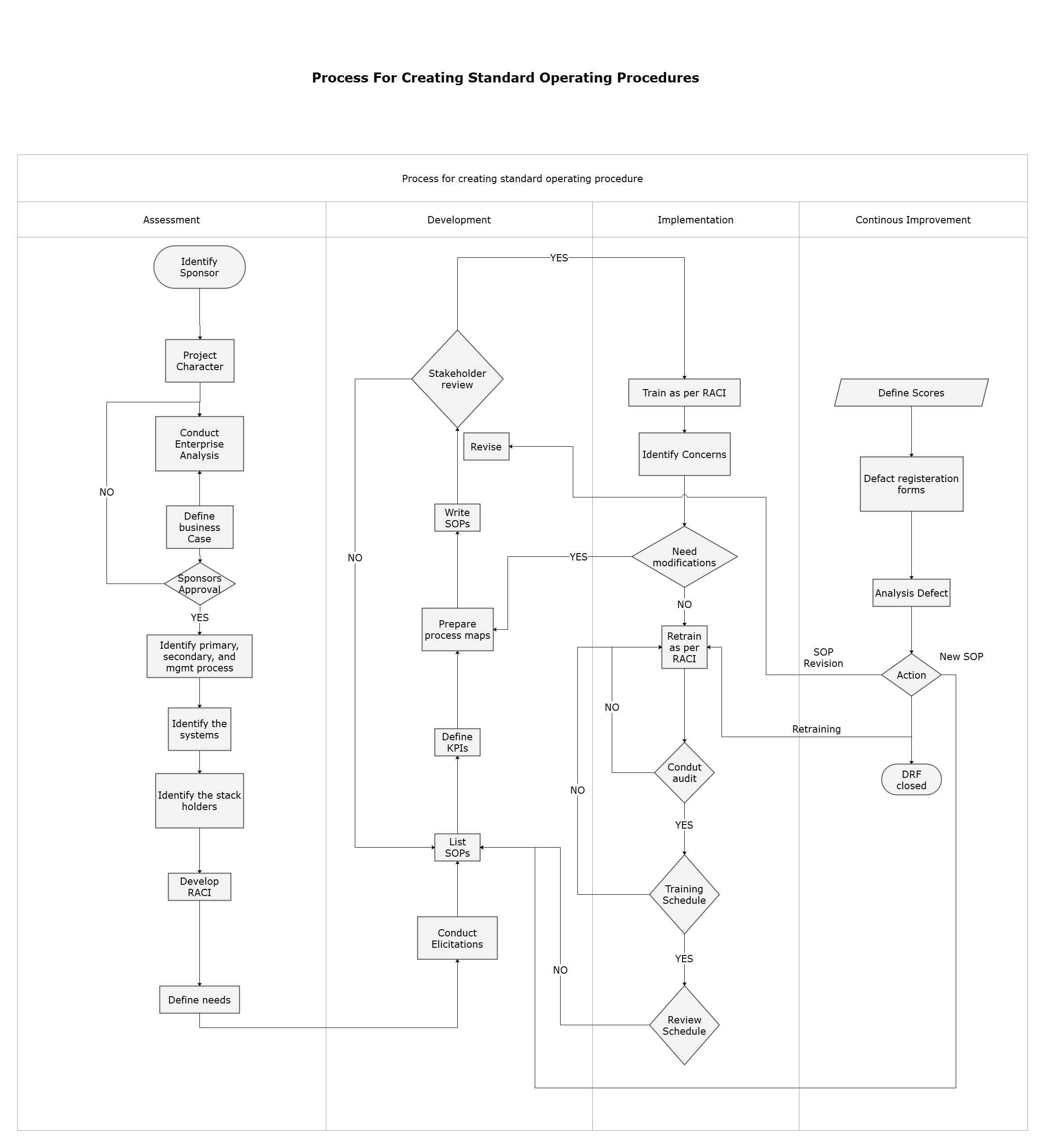
How To Create A Standard Operating Procedure Edrawmax Porn Sex Picture
Enpharmaceutical Sop Template
1 Purpose This SOP defines the actions and responsibilities for the handling of reports of suspected defective centrally authorised medicinal products received by the EMA Secretariat and which may require immediate action This SOP applies to all defective product reports and to all reported product quality problems received by any EMA staff
Calibration SOPs Production SOP QC SOP QA SOP SOP for cleaning Microbiology SOP SOP lists for all departments
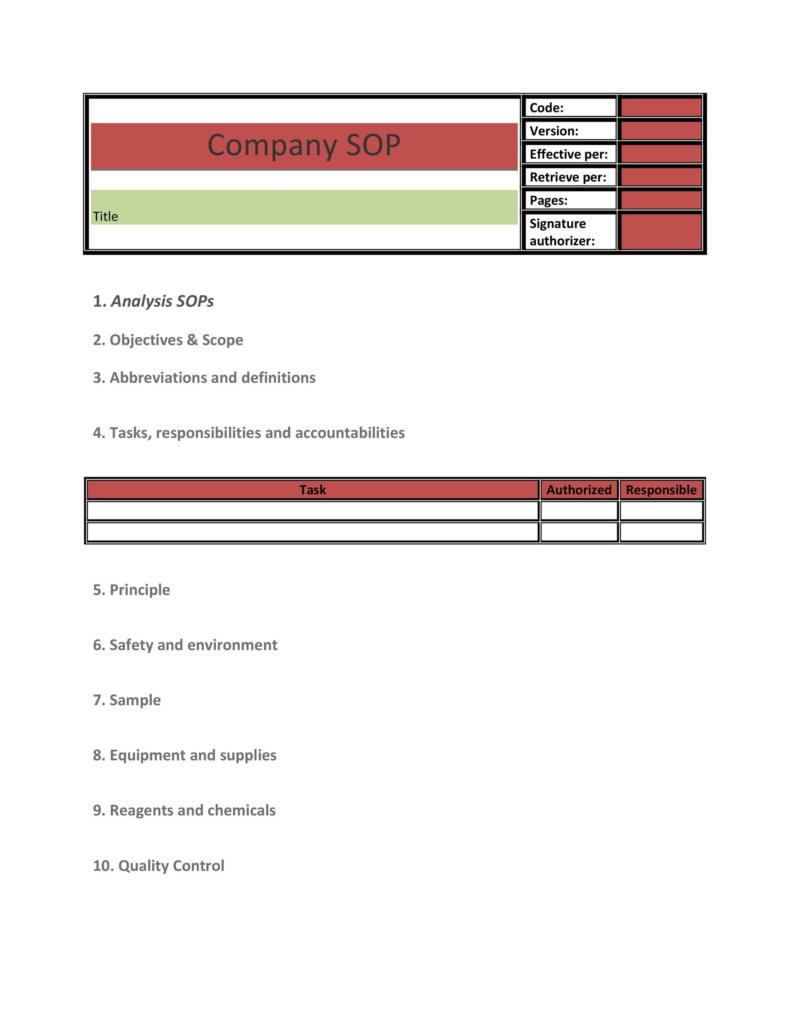
37 Best Standard Operating Procedure SOP Templates

How To Write A Standard Operating Procedure SOP Example ProcessDriven

37 Best Standard Operating Procedure SOP Templates Tap The Link Now

37 Best Standard Operating Procedure SOP Templates

Standard Operating Procedure Template SOP Template Editable Word Form