Enphase 1 Clinical Trial Protocol Template - This paper details guidelines for the content of statistical analysis plans SAPs for early phase clinical trials presenting an extension to the guidelines for the content of SAPs in clinical trials by Gamble et al 1 Early phase clinical trials phase I and non randomised phase II aim to determine the safety and initial indicators of efficacy of interventions before conducting phase III
Study No TAK 653 1001 Page 3 of 126 Protocol Incorporating Amendment No 06 24 March 2017 CONFIDENTIAL 1 2 Approval REPRESENTATIVES OF TAKEDA This study will be conducted with the highest respect for the individual participants in accordance with the requirements of this clinical study protocol and also in accordance with the following
Enphase 1 Clinical Trial Protocol Template

Enphase 1 Clinical Trial Protocol Template
US Clinical Development and Medical Affairs . AIN457/Secukinumab . Clinical Trial Protocol CAIN457FUS06 / NCT03350815. A randomized, double-blind, parallel-group, multicenter study of secukinumab to compare 300 mg and 150 mg at Week 52 in patients with Ankylosing Spondylitis who are randomized to dose escalation after not achieving inactive
Final Protocol Amendment 25 11May2021 PFIZER CONFIDENTIAL Page 2 Document History Document Version Date Summary of Changesand Rationale Amendment 25 11May2021 Sections 5 2 1 1 5 3 and Appendix 11 updated to allow usage of the PF 02341066 formulated capsules Japan specific amendment Amendment 24 26 March 2019 Sections4 3 and 5 5 1 updated
Span Class Result Type
Generic Protocol Template MS Word updated August 4 2023 Generic Informed Consent Template NCI Informed Consent Template for CTEP Trials MS Word This is a Generic NCI Template with a blank Summary of Changes cover memo for CTEP submission Protocol Template for Organ Dysfunction Studies MS Word updated August 4 2023

Clinical Protocol Template TUTORE ORG Master Of Documents
This trial protocol has been provided by the authors to give readers additional information about their work vaccine candidates N Engl J Med 2020 383 2439 50
Nih Protocol Template TUTORE ORG Master Of Documents

Clinical Study Protocol PowerPoint And Google Slides Template PPT Slides
Early Phase Clinical Trials Extension To Guidelines For The Content Of
The clinical trials electronic protocol writing template tool provides a useful format for NIH funded Phase II and III clinical trials that are being conducted under an FDA Investigational New Drug IND or Investigational Device Exemption IDE Application The goal of the template is to help investigators think through the scientific basis of their assumptions minimize uncertainty in the

Clinical Protocol Template TUTORE ORG Master Of Documents
4 Brochure E Protocols 21 CFR 312 23 a 6 The regulation requires submission of a copy of the protocol for the conduct of each proposed clinical trial
This document provides the template for the clinical study protocol, a key document in the planning and conduct of clinical trials. It follows the ICH M11 guideline and the technical specifications for data exchange. The template aims to facilitate the preparation and review of protocols and to promote harmonisation across regions.
Span Class Result Type
The clinical trials electronic protocol writing template tool provides a useful format for NIH funded Phase II and III clinical trials that are being conducted under an FDA Investigational New Drug IND or Investigational Device Exemption IDE Application The goal of the template is to help investigators think through the scientific basis of their assumptions minimize uncertainty in the

Clinical Trial Protocol Template
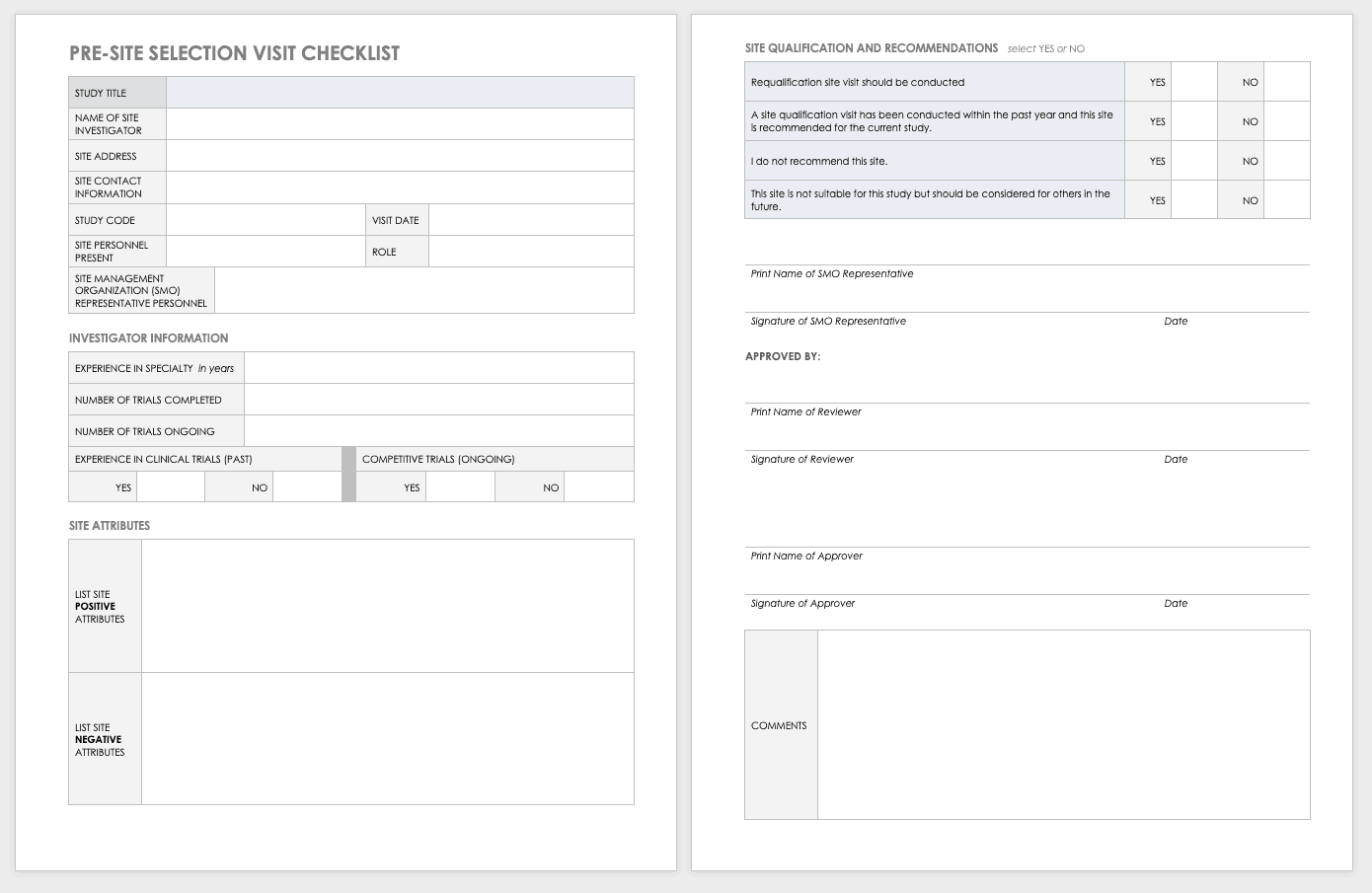
Monitoring Report Template Clinical Trials
Enphase 1 Clinical Trial Protocol Template
4 Brochure E Protocols 21 CFR 312 23 a 6 The regulation requires submission of a copy of the protocol for the conduct of each proposed clinical trial
Study No TAK 653 1001 Page 3 of 126 Protocol Incorporating Amendment No 06 24 March 2017 CONFIDENTIAL 1 2 Approval REPRESENTATIVES OF TAKEDA This study will be conducted with the highest respect for the individual participants in accordance with the requirements of this clinical study protocol and also in accordance with the following

Free Clinical Trial Templates Smartsheet

Phase 1 Clinical Trial Protocol Template Templates MTE5NTAx Resume
Clinical Trial Protocol Template Ich
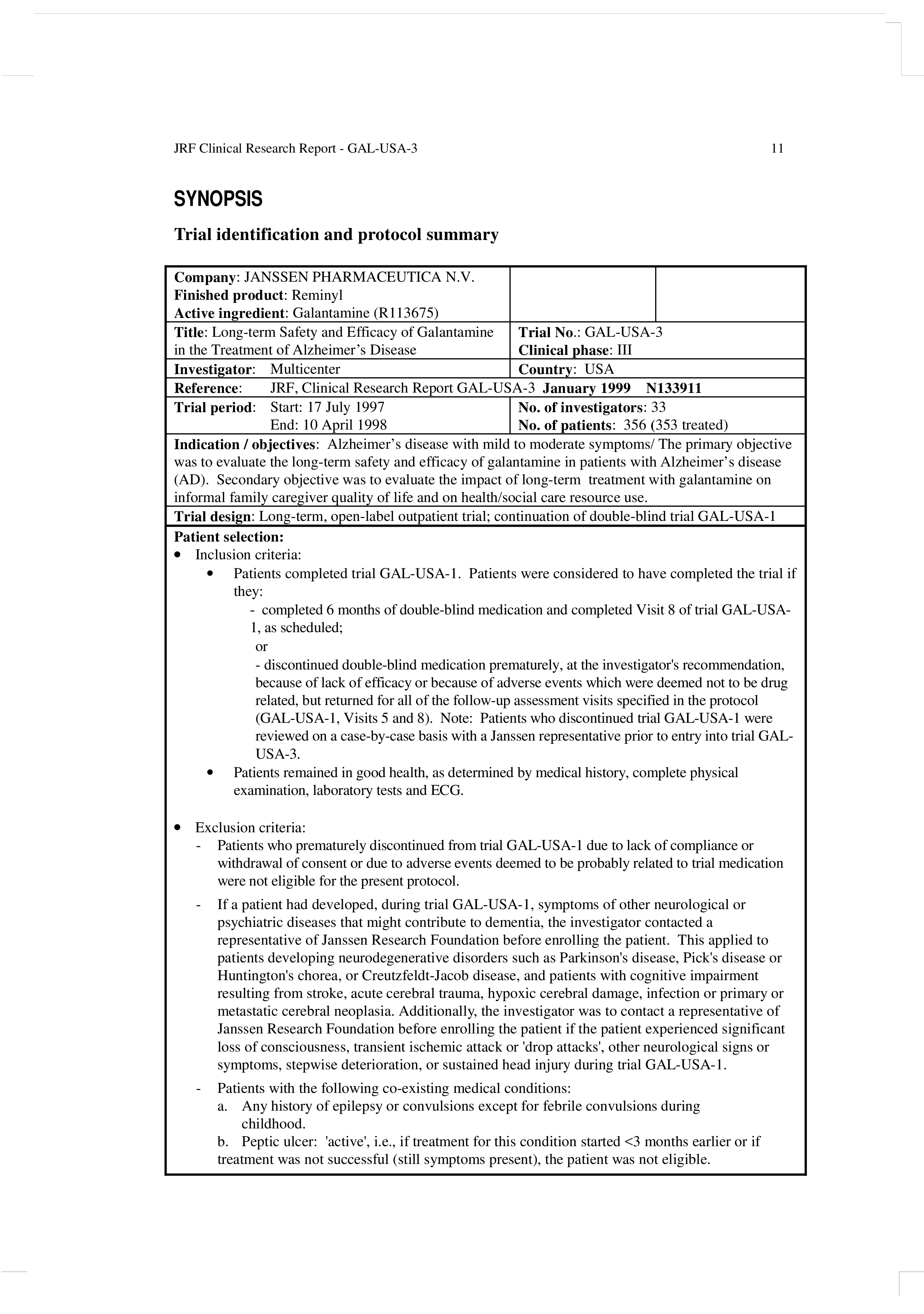
Clinical Protocol Template TUTORE ORG Master Of Documents

Clinical Trial Protocol