Enregulatory Strategy Template For Medical Devices - Now in almost all markets worldwide the device s risk classification determines the level of regulatory scrutiny To classify a medical device incl in vitro diagnostic devices IVDs the manufacturer must have established its intended purpose and intended user as well as a comprehensive list of device functions Therefore even if it is important to have an overview of the regulatory
Develop a go to market strategy early Developing a detailed strategy early in the product development process can identify the options offering the best ROI while providing a roadmap for success Study the regulatory compliance landscape There are commonalities in regulatory requirements but also important differences
Enregulatory Strategy Template For Medical Devices
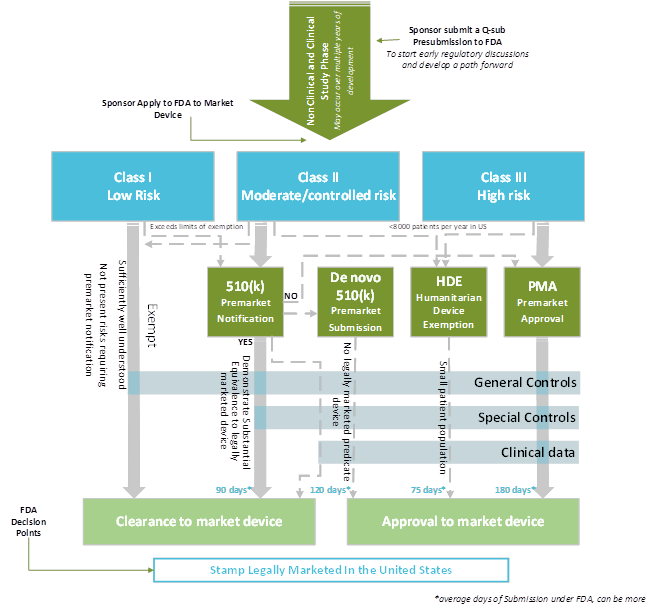
Enregulatory Strategy Template For Medical Devices
Your regulatory strategy is the process by which you end up with a pathway to market. This difference is important because you almost always have multiple options for the market pathway you'll use to get your device on the market. The decision between using the 510 (k) pathway and Pre-Market Approval (PMA) in the US, for instance, is not as ...
Hi pbojsen You include the following topics in the Reg Plan 1 Introduction of the Medical Device 2 Intended USe with photo of the device 3 Regulatory strategy in which countries you want to market first or priorities either RUO or IVD by when is it in phases or parallel launch of the product etc 3 1 Regulatory strategy A US a Overview b Intended USe c Scope in scope
How To Build A Global Regulatory Strategy For Medical MasterControl
Build an Effective Global Regulatory Strategy Getting through the complex maze of regulatory requirements can be an overwhelming process for any medical device company For medical device manufacturers gaining access to multiple major markets is important for the success of the company particularly when the product lines involve new or novel
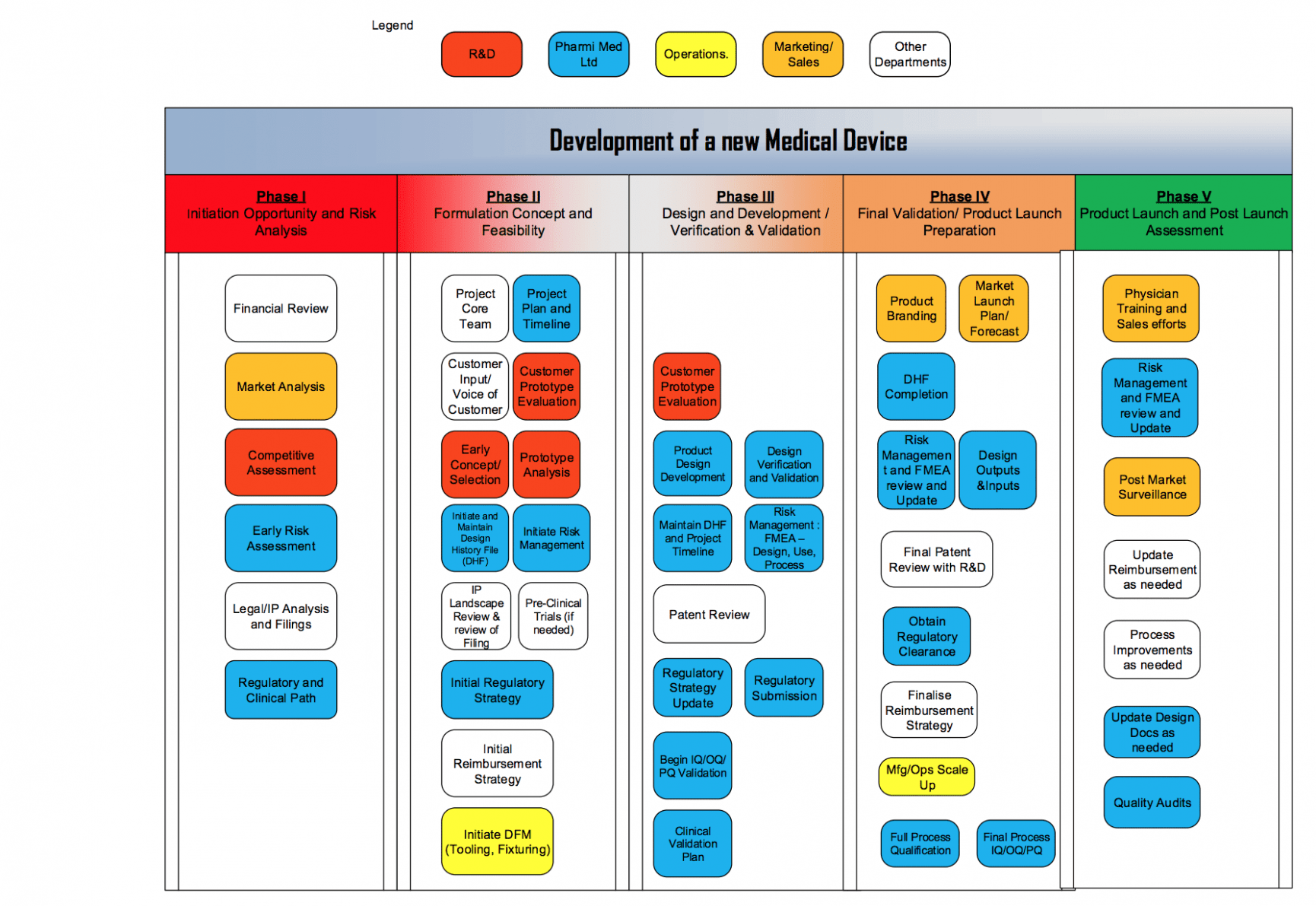
Medical Device Design And Development Plan Template
2 with respect to medical products is to develop the knowledge methods standards and tools needed to increase the certainty and consistency of regulatory decisions and improve the translation

FDA On Reusable Medical Devices And Reprocessing RegDesk

Medical Release Authorization Form Template Addictionary
Medical Device Regulatory Strategy US Vs EU Decomplix
Step 2 Identify your healthcare claim and product label Step 5 Establish your healthcare product development plan Step 6 Execute your healthcare product development plan Healthcare product development Step 4 Develop your regulatory strategy When developing a healthcare product keep your regulatory strategy well balanced and realistic

Clinical Nurse Clinical Research Planner Template Brochure Template
The strategy for regulatory compliance would seem to be the obvious place to document the regulatory role or roles of your organization The strategy for regulatory compliance is part of the QMS Just like all other QMS processes it has to be maintained i e kept up to date As well as being a regulatory requirement establishing the
The need for a regulatory strategy stems from Article 10 (9a) of the EU Medical Device Regulation (MDR) or Article 10 (8a) of the In Vitro Diagnostic Medical Devices Regulation (IVDR) which states: "The quality management system shall address at least the following aspects: (a) a strategy for regulatory compliance, including compliance with ...
Planning Your Medical Device Global Market Regulatory Strategy
So a regulatory strategy and this is actually the Oxford Dictionary s definition of strategy which I like is a plan of action designed to achieve a major goal or objective a plan of action designed to achieve a major goal or objective So a 510 K or a PMA is your objective but it s not your plan on how you get there so that s I think

ISO 14971 Risk Management For Medical Devices

Medical Release Authorization Form Template Addictionary
Enregulatory Strategy Template For Medical Devices
The strategy for regulatory compliance would seem to be the obvious place to document the regulatory role or roles of your organization The strategy for regulatory compliance is part of the QMS Just like all other QMS processes it has to be maintained i e kept up to date As well as being a regulatory requirement establishing the
Develop a go to market strategy early Developing a detailed strategy early in the product development process can identify the options offering the best ROI while providing a roadmap for success Study the regulatory compliance landscape There are commonalities in regulatory requirements but also important differences
This Is A Partial Preview Of Training Plan Template

Regulatory Affairs For Medical Devices Level 2

Regulatory Affairs For Medical Devices Level 1

Medical Release Authorization Form Template Addictionary

Addictionary