Enrt Template Health Canada - For industry information about COVID 19 visit our COVID 19 Drugs and vaccines section Guidance documents have been prepared to assist in the interpretation of policies and governing statutes and regulations They are intended to assist in preparing drug submissions when seeking an approval to sell a pharmaceutical drug product in Canada A B
Templates Biopharmaceutics Classification System BCS based Biowaiver Evaluation template 2020 08 26 A blank Foreign Review Attestation template is available in Microsoft Word upon request To receive a blank Foreign Review Attestation template please email policy bureau enquiries hc sc gc ca
Enrt Template Health Canada
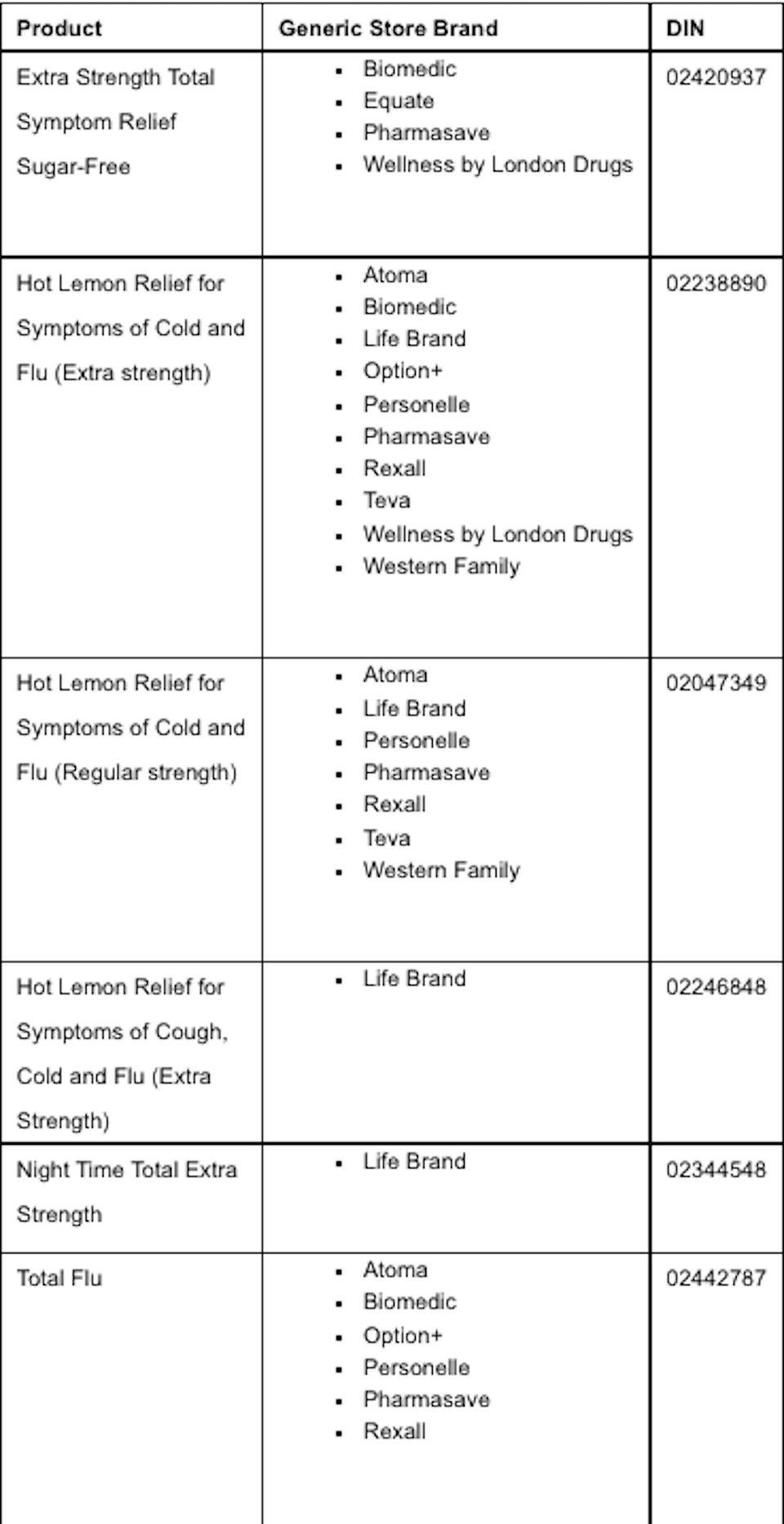
Enrt Template Health Canada
The "Company Identifier" field will be filled by Health Canada when they generate the final company XML file." The "Amend Enrolment" button is only enabled when a final company xml file is loaded into the template, and must be selected when amending a final company XML file." Return to instruction reference 2. Instruction 3. Reason for ...
Regulatory Transaction RT Template updated on 2023 02 28 Required with each regulatory transaction filed to Health Canada Sent via the CESG in folder 1 2 1 for human drugs and disinfectants or part I folder 1 5 for veterinary drugs of the regulatory transaction submission
Templates Canada Ca
Yes No Fees 15 Do not send fee payment with your submission supplement or application Health Canada will verify the fee and issue an invoice accordingly For information on payment of invoices and current fees please visit the Fees and Funding webpage Submission Class required Submission Description Mitigation Measures

Health Canada On Classification Of Non IVD Medical Devices RegDesk
To use an electronic form you must first download the form to your computer and then use Adobe Reader or Adobe Acrobat software latest version to successfully complete the PDF fillable form The Adobe Reader software is available for free on the Adobe website
Health Canada Prepares For New Labeling Regulations MEAT POULTRY

Health Canada Front Of Package FOP Labeling Regulatory Session YouTube
Applications And Submissions Drug Products Canada Ca
2013 04 03 Publication information bibliographic Record

Honoured To Be On The List Of Canada s Top 100 Health Influencers
1 Introduction 1 1 Scope and Application This guidance document provides market authorization holders MAHs with information on how to comply with the Food and Drugs Act the Food and Drug Regulations and the Natural Health Products Regulations with respect to preparing and submitting annual summary reports ASRs and issue related summary reports IRSRs
Author: Preetha Prabhu. On Mar. 30, 2020, Health Canada published a notice that as of October 1, 2020 use of the Regulatory Enrolment Process (REP) will be mandatory for pharmaceutical, biologic and radiopharmaceutical drugs for human use as well as disinfectants, pursuant to Part C, Division 1 and Division 8 of the Food and Drug Regulations.
Company Template Regulatory Enrolment Process Canada Ca
2 1 Cover letter 2 2 Folder structure and file naming convention 2 2 1 Top level folder dossier identifier 2 2 2 Common Technical Document CTD folder structure 2 2 3 Veterinary drugs folder structure 2 3 File naming convention 3 Technical requirements for regulatory activities 3 1 File format 3 2 Signatures 3 3 Validation of transaction

Health Canada Approves INREBIC fedratinib First New Treatment In
Health Canada Approves Pfizer Anti viral Pill For Treatment Of COVID 19
Enrt Template Health Canada
1 Introduction 1 1 Scope and Application This guidance document provides market authorization holders MAHs with information on how to comply with the Food and Drugs Act the Food and Drug Regulations and the Natural Health Products Regulations with respect to preparing and submitting annual summary reports ASRs and issue related summary reports IRSRs
Templates Biopharmaceutics Classification System BCS based Biowaiver Evaluation template 2020 08 26 A blank Foreign Review Attestation template is available in Microsoft Word upon request To receive a blank Foreign Review Attestation template please email policy bureau enquiries hc sc gc ca

Health Canada 2008 2009 Report On Plans And Priorities

Health Canada Looking For Volunteers For Help With COVID 19 Alberta
PDF On What Basis Did Health Canada Approve OxyContin In 1996 A

Health Canada Issues Notice Of Compliance For TRODELVY A New

Health Canada 2006 2007 REPORT ON PLANS AND PRIORITIES