Fda 1571 Form 2024 - Form 1571 Investigational New Drug Application IND form Form 356h Form for New Drug Applications NDAs and Biologics License Applications BLAs Both forms represent several changes and add additional information to help the FDA s Central Document Room and reviewer evaluate and route the submissions Form FDA 1571
Investigational New Drug Application Instructions for completing Form FDA 1571 FDA 1572 PDF 1 4MB Statement of Investigator Instructions for completing Form FDA 1572 FDA 3454 PDF
Fda 1571 Form 2024
Fda 1571 Form 2024
En Español. Radiation-Emitting Products. Reports, Manuals, & Forms. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Use the ...
Additional Resources Form FDA 356h Form FDA 356h Instructions Form FDA 1571 Form FDA1571 Instructions Abbreviated New Drug Application ANDA Forms and Submission Requirements
Clinical Trial Forms FDA U S Food And Drug Administration
Form FDA 1571 and 1572 are still required for other expanded access submissions e g intermediate access or treatment INDs and for IND submissions by commercial sponsors or drug
Free Printable Oir B1 1571 Form Printable Forms Free Online
August 7 2023 The FDA has announced several revisions to its Investigational New Drug IND application Form 1571 intended to improve data quality and usability The changes are as follows Form Field 1 Added new checkbox to indicate the FDA center the application is intended for CDER or CBER

2003 Form FL OIR B1 1571 Fill Online Printable Fillable Blank PdfFiller

1571 Form Fill Out And Sign Printable PDF Template SignNow
New FDA Forms For INDs NDAs And BLAs What To Know Before Submitting
What is the Form Used For Cover sheet for Investigational New Drug Application IND submissions Indicates what is being provided in the submission Captures information tracked by FDA systems Why Was the Form Updated Need for additional tracking e g rare disease information

PPT Investigational New Drug IND Orientation Responsibilities Of IND Sponsor Investigators
Certain important commitments that the IND sponsor makes by signing Form FDA 1571 are listed below field 16 Under Section 744G 11 of the Federal Food Drug and Cosmetic Act the FD C Act as added by the Biosimilar User Fee Act of 2012 BsUFA the term financial hold means an order issued by FDA to prohibit
Form FDA 1571 Instructions. The purpose of the Form FDA 1571 is to: obtain agreement from the sponsor (or sponsor-investigator) to conduct research according to all appropriate FDA regulations; and; serve as a cover sheet for all submissions to the FDA on behalf of a particular IND. Form FDA 1571 should be completed for every submission sent to ...
Forms FDA U S Food And Drug Administration
An Investigational New Drug IND application is a request for FDA to administer an investigational drug to humans Sponsors submitting INDs should include Form 1571 For guidance on Form 1571 and to download a fillable PDF select this link To apply to market a new drug biologic or an antibiotic drug for human use you need to complete
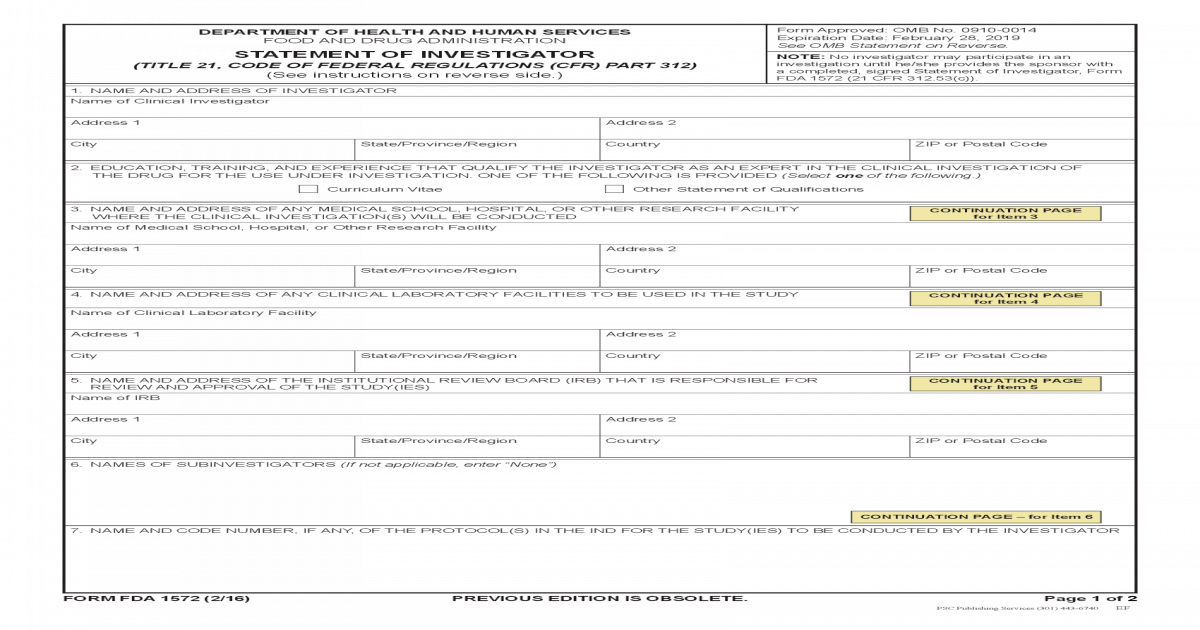
Form FDA 1572 PDF 208KB PDF Document

PPT An FDA Audit What The Investigator And Sponsor Need To Know PowerPoint Presentation ID
Fda 1571 Form 2024
Certain important commitments that the IND sponsor makes by signing Form FDA 1571 are listed below field 16 Under Section 744G 11 of the Federal Food Drug and Cosmetic Act the FD C Act as added by the Biosimilar User Fee Act of 2012 BsUFA the term financial hold means an order issued by FDA to prohibit
Investigational New Drug Application Instructions for completing Form FDA 1571 FDA 1572 PDF 1 4MB Statement of Investigator Instructions for completing Form FDA 1572 FDA 3454 PDF

FDA Voluntary Consensus Standards RegDesk

Free Printable Oir B1 1571 Form Printable Forms Free Online
FDA 1572 Institutional Review Board Health Sciences
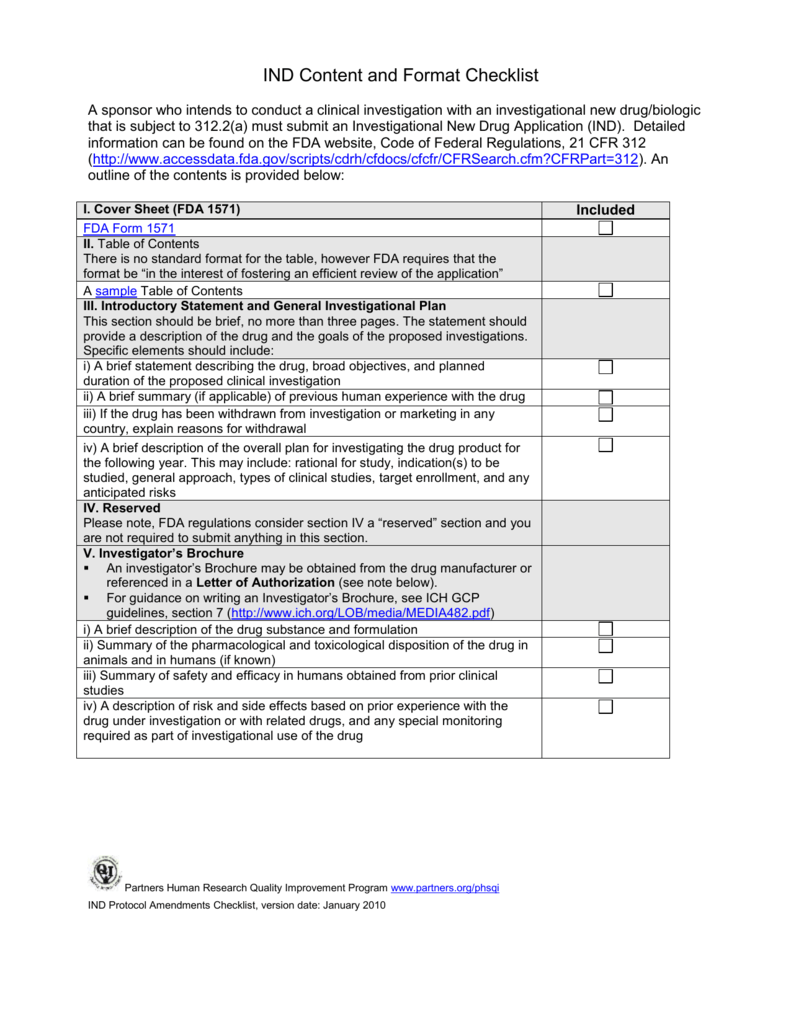
IND Annual Report Template

FDA Registered And Inspected Facility Seal

