Fda 1572 Form 2024 - The Food and Drug Administration FDA Agency or we is announcing the availability of a draft information sheet guidance for sponsors clinical investigators and institutional review boards IRBs entitled Frequently Asked Questions Statement of Investigator Form FDA 1572 Revision 1
The Food and Drug Administration FDA or agency has received a number of questions about Form FDA 1572 The most frequently asked questions are answered below If you do not see your
Fda 1572 Form 2024

Fda 1572 Form 2024
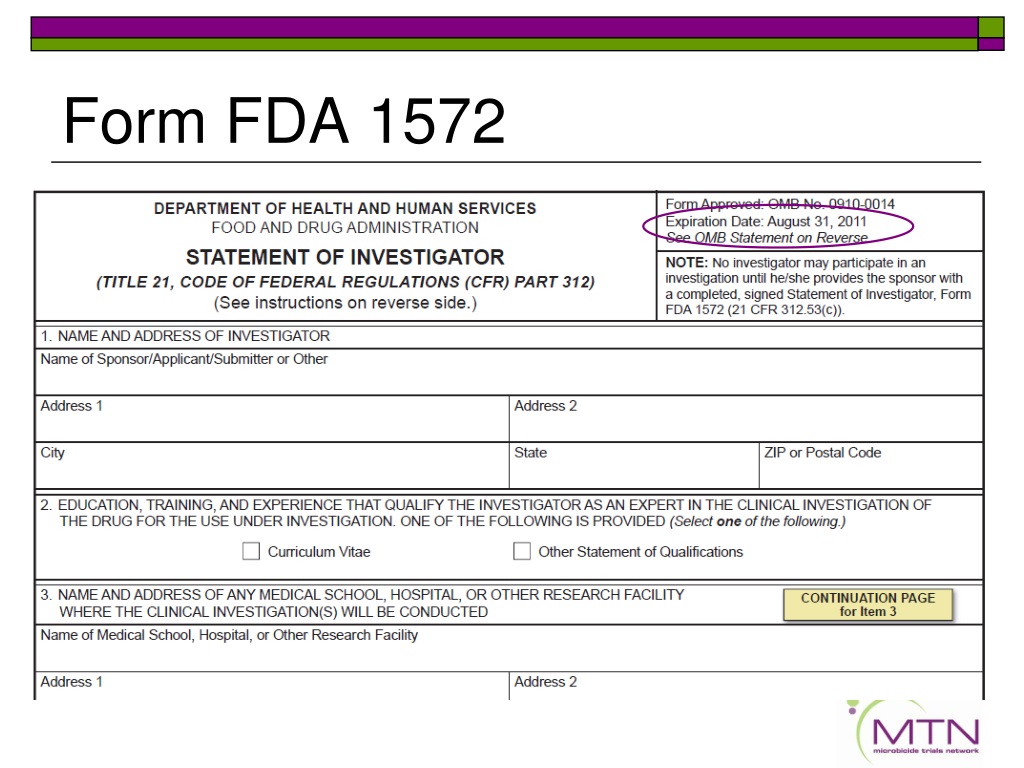
The Statement of Investigator, Form FDA 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will
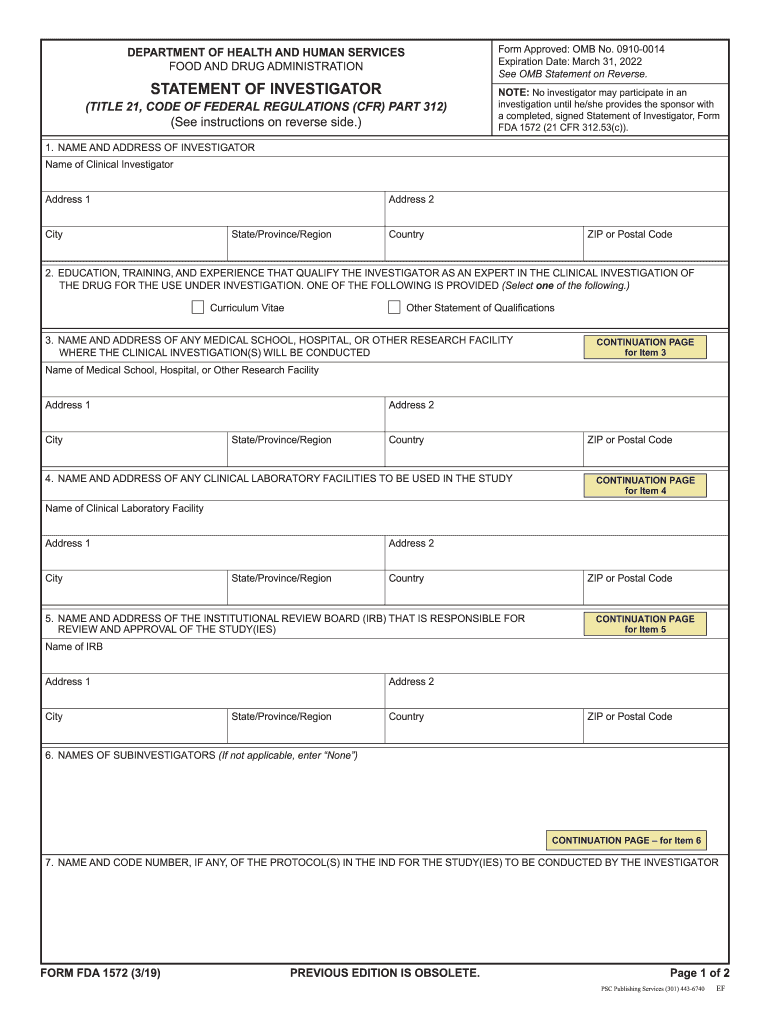
Investigational New Drug Application Instructions for completing Form FDA 1571 FDA 1572 PDF 1 4MB Statement of Investigator Instructions for completing Form FDA 1572 FDA 3454
Frequently Asked Questions Statement Of Investigator Form FDA 1572
Form FDA 1571 and 1572 are still required for other expanded access submissions e g intermediate access or treatment INDs and for IND submissions by commercial sponsors or drug
PPT INVESTIGATOR RESPONSIBILITIES PowerPoint Presentation Free Download ID 9351214
Appendix C 1572 Related Excerpts from Good Clinical Practice A Question Answer Reference Guide 2008 License Format Properties Price Paperback The book paperback will be shipped to you 43 EUR 45 USD 37 GBP The Form FDA 1572 A Reference Guide for Clinical Researchers Sponsors and Monitors
FDA 1572 Institutional Review Board Health Sciences

Investigator Statement FDA 1572 Centers For Doc Template PdfFiller
Frequently Asked Questions Statement Of Investigator Form FDA 1572
An FDA Form 1572 is required for drug or biologic studies conducted under an IND whether in the U S or abroad Similarly medical device trials require a signed agreement from each investigator that contains information similar to that requested in the 1572 There has been long standing confusion and disagreement over who should be listed as
Fda 1572 Continuation Page 4 2019 2023 Form Fill Out And Sign Printable PDF Template SignNow
On 20 May 2021 the FDA released a draft information sheet guidance for sponsors clinical investigators and institutional review boards IRBs entitled Frequently Asked Questions Statement of Investigator Form FDA 1572 Revision 1
The FDA has updated its guidance on Form 1572, the FDA's Statement of Investigator, to clarify what sponsors can or should do if a foreign investigator is unable or unwilling to sign the form. The guidance was developed in response to sponsor questions on how to proceed if regional, national or local laws or regulations prohibit a foreign investigator from signing the form.
Information Sheet Guidance For Sponsors Clinical Investigators And IRBs
By signing Form FDA 1572 Form 1572 the investigator of a drug or biologic trial warrants that they and any listed staff have the experience and background needed to conduct the trial and agrees to comply with the protocol and all applicable U S regulatory provisions governing the conduct of clinical trials
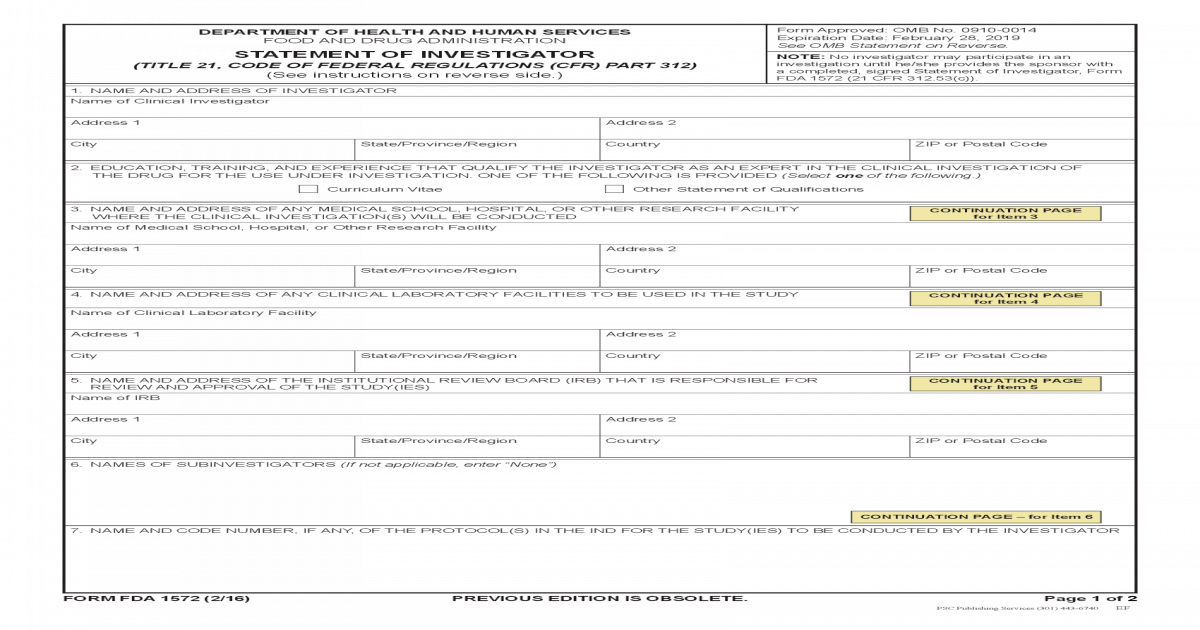
Form FDA 1572 PDF 208KB PDF Document

Fda 1572 Form Fill Out Printable PDF Forms Online
Fda 1572 Form 2024
On 20 May 2021 the FDA released a draft information sheet guidance for sponsors clinical investigators and institutional review boards IRBs entitled Frequently Asked Questions Statement of Investigator Form FDA 1572 Revision 1
The Food and Drug Administration FDA or agency has received a number of questions about Form FDA 1572 The most frequently asked questions are answered below If you do not see your

The Investigator s Guide To Form FDA 1572 Getting The Statement Of Investigator Right CenterWatch

Form FDA 1572 YouTube
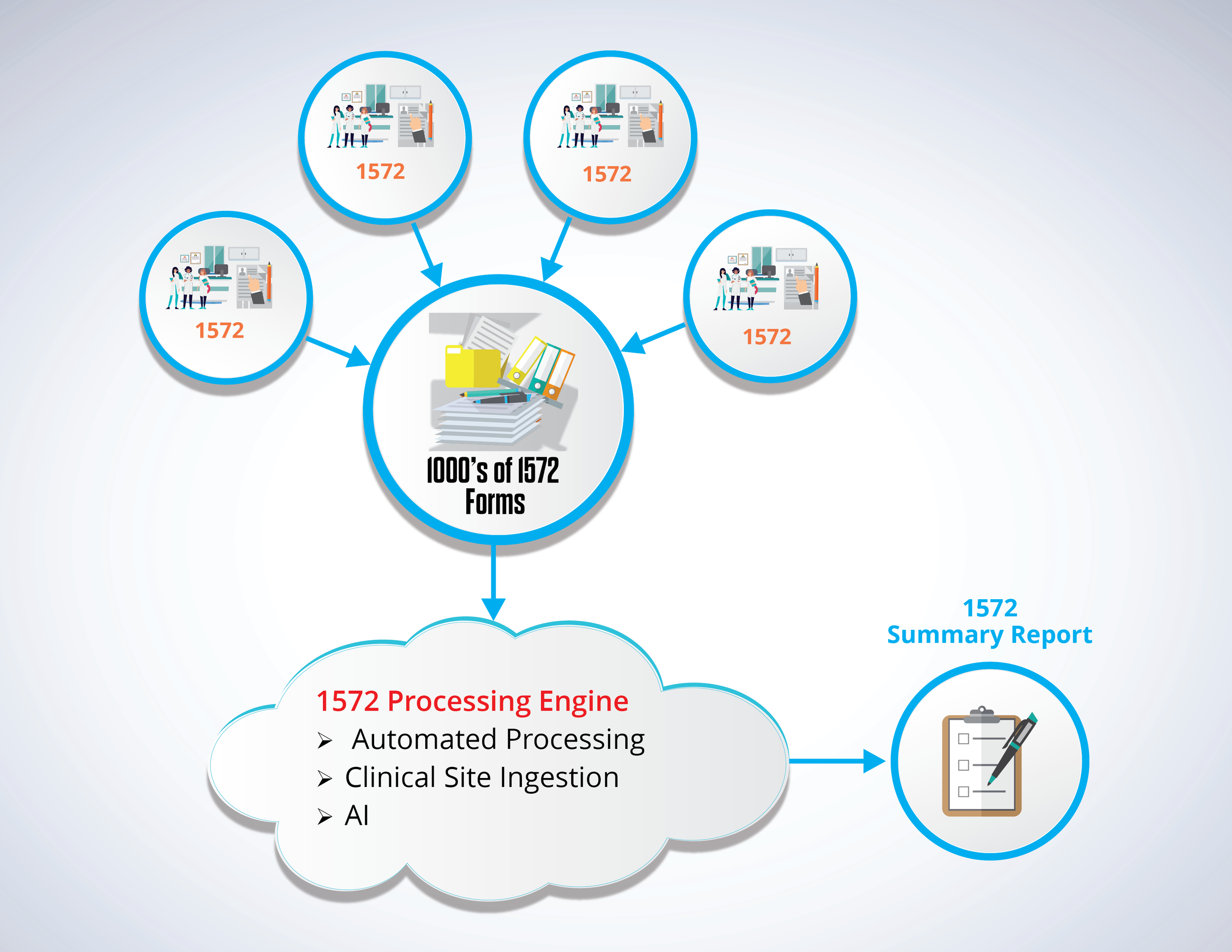
Streamlined Solution For FDA Form 1572 Summary Report Processing RegDocs365

FDA 1572 Instructional Supplement Food And Drug Administration Doc Template PdfFiller

Food Drug Administration Reconnaissez Le Parfait Formulaire
