Fda Pre Submission Template - ESTAR is the only available electronic submission template to prepare 510 k electronic submissions eSTAR is an interactive PDF form that guides applicants through the process of preparing
How to use the electronic Submission Template And Resource eSTAR PDF template Send and Track Medical Device Premarket Submissions Online CDRH Portal
Fda Pre Submission Template

Fda Pre Submission Template
Some highlights of this episode include: A Pre-submission meeting is an opportunity to communicate with FDA prior to a marketing submission. About 3,306 medical device-related Pre-submission requests were made to FDA in 2020. In 2021, more than 1,500 Pre-submission requests have been made so far.
The template is only used for constructing not submitting your submission The directions at the end of the template provide instructions on how to submit File Size Processing of the
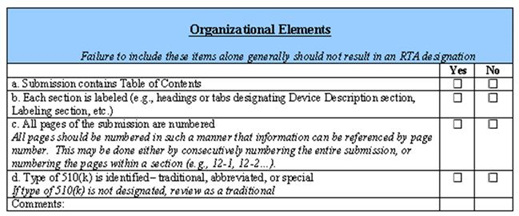
Premarket Submissions Selecting And Preparing The Correct Submission FDA
This guidance is intended to represent one of several steps in meeting FDA s commitment to the development of electronic submission templates to serve as guided submission preparation tools

Fda Annual Report Cover Letter Template Online Cover Letter Library
The idea of preparing for a Pre Submission meeting with the FDA can be a stressful thought You may not even know where to begin Luckily we have organized a simple yet detailed guide to help you get started as well as provide you with free downloadable tools to develop your roadmap
FDA
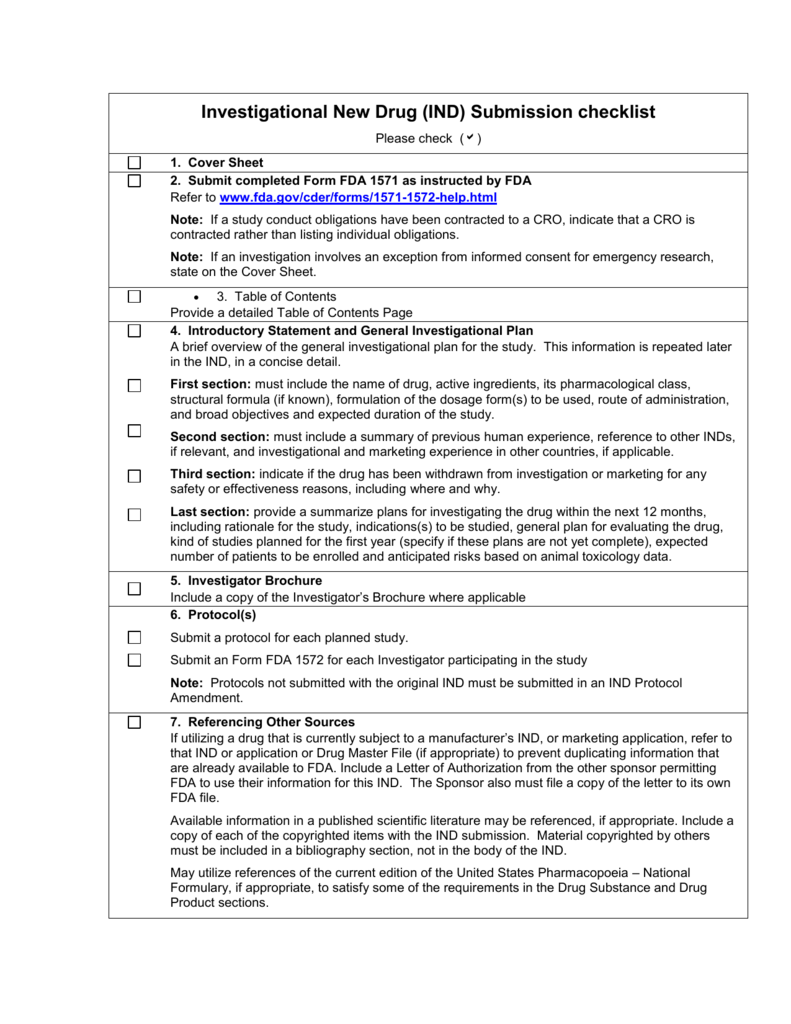
Investigational New Drug IND Submission Checklist
ESTAR Program FDA U S Food And Drug Administration
Clearly indicate what type of future submission i e IDE 510 k etc is the focus of your Pre Sub questions to help direct FDA s feedback Requests for feedback regarding study design for non significant risk NSR or IDE exempt studies for which the results are not expected to support a future IDE or marketing application

A Quick Easy Guide To FDA Pre Submissions
FDA Forms and Electronic Submissions Forms Official FDA applications and submissions forms Electronic Regulatory Submission and Review Information about review and electronic submission
An FDA pre-submission aims to get answers to questions you have about a future FDA submission. The pre-submission may consist of one large PDF document or multiple PDF documents. In your pre-submission, you must select either an email response or an email response with a teleconference.
Preparing Your Pre Submission With The Content FDA Wants To See
For Medical Device Submissions The Q Submission Program Guidance for Industry and Food and Drug Administration Staff Document issued on June 2 2023 Document originally issued on May 7

Why The FDA Pre Submission Is An Underutilized Tool

How To Prepare For And Make The Most Out Of Your FDA Pre Submission
Fda Pre Submission Template
FDA Forms and Electronic Submissions Forms Official FDA applications and submissions forms Electronic Regulatory Submission and Review Information about review and electronic submission
How to use the electronic Submission Template And Resource eSTAR PDF template Send and Track Medical Device Premarket Submissions Online CDRH Portal
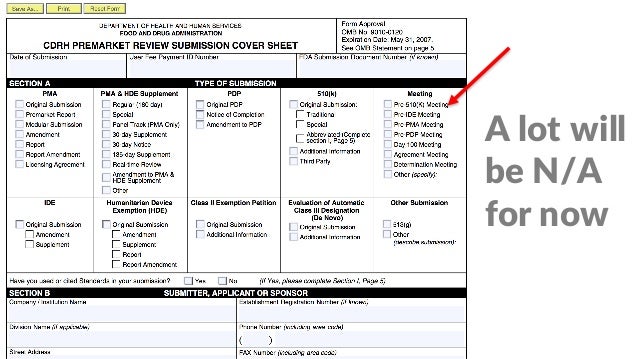
How To Prepare For And Make The Most Out Of Your FDA Pre Submission

FDA 510 k Submission Redacted

Why The FDA Pre Submission Is An Underutilized Tool
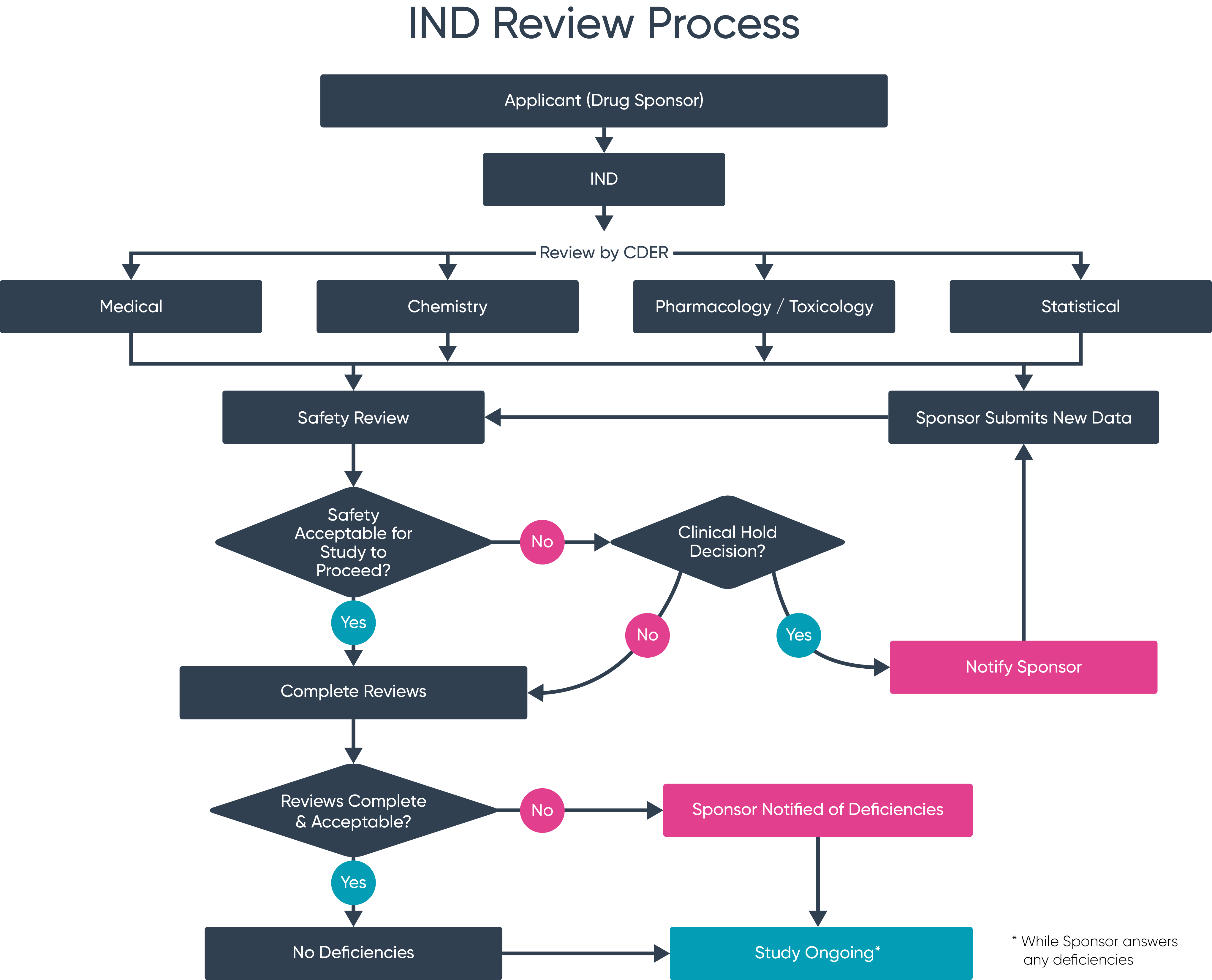
Pre IND Meeting FDA Your Need to knows Ideagen
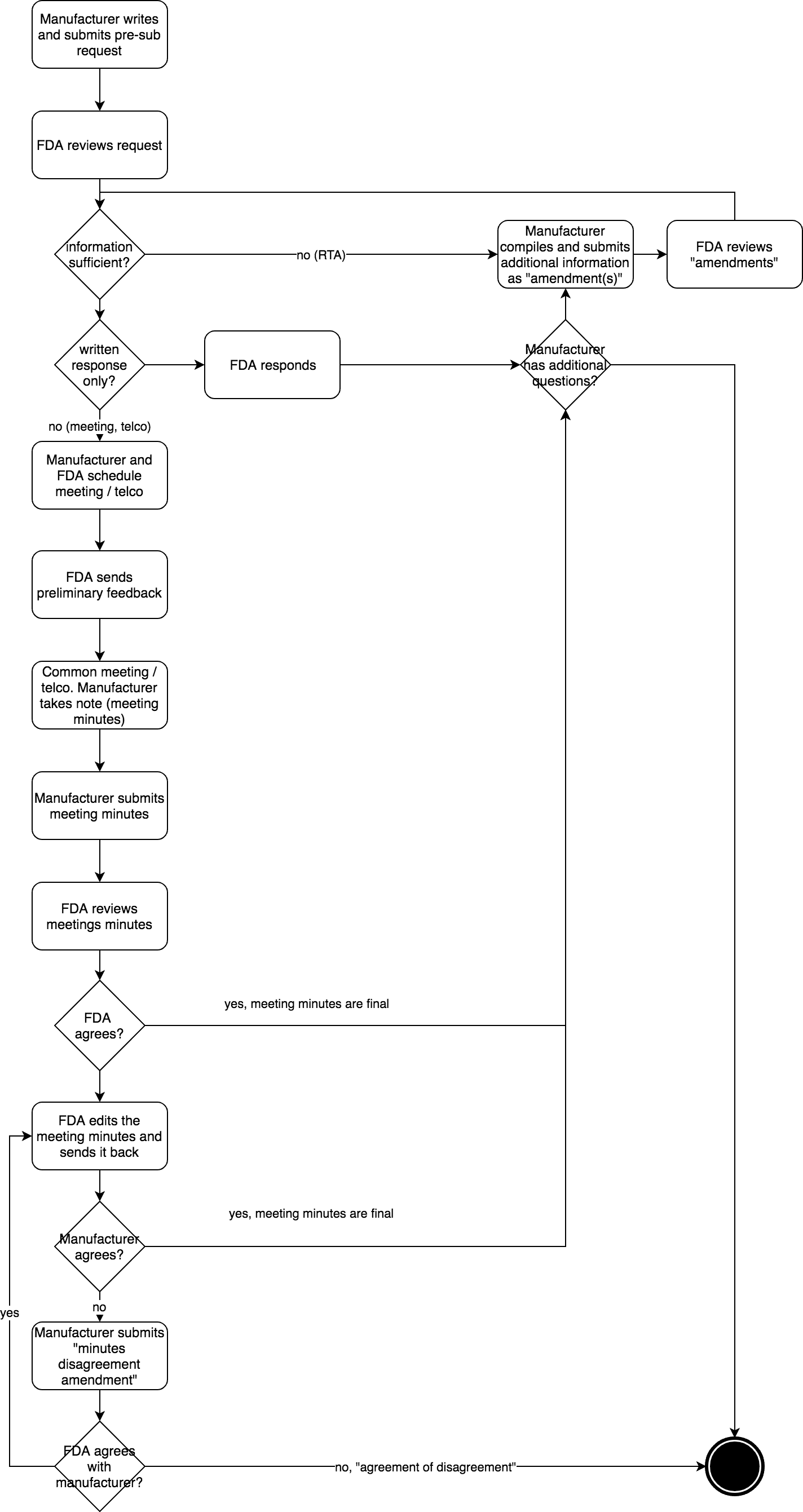
FDA Pre Submission Programm