Ionic Bonds Form - Anion A negatively charged ion Ionic Bonds Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another This exchange results in a more stable noble gas electronic configuration for both atoms involved
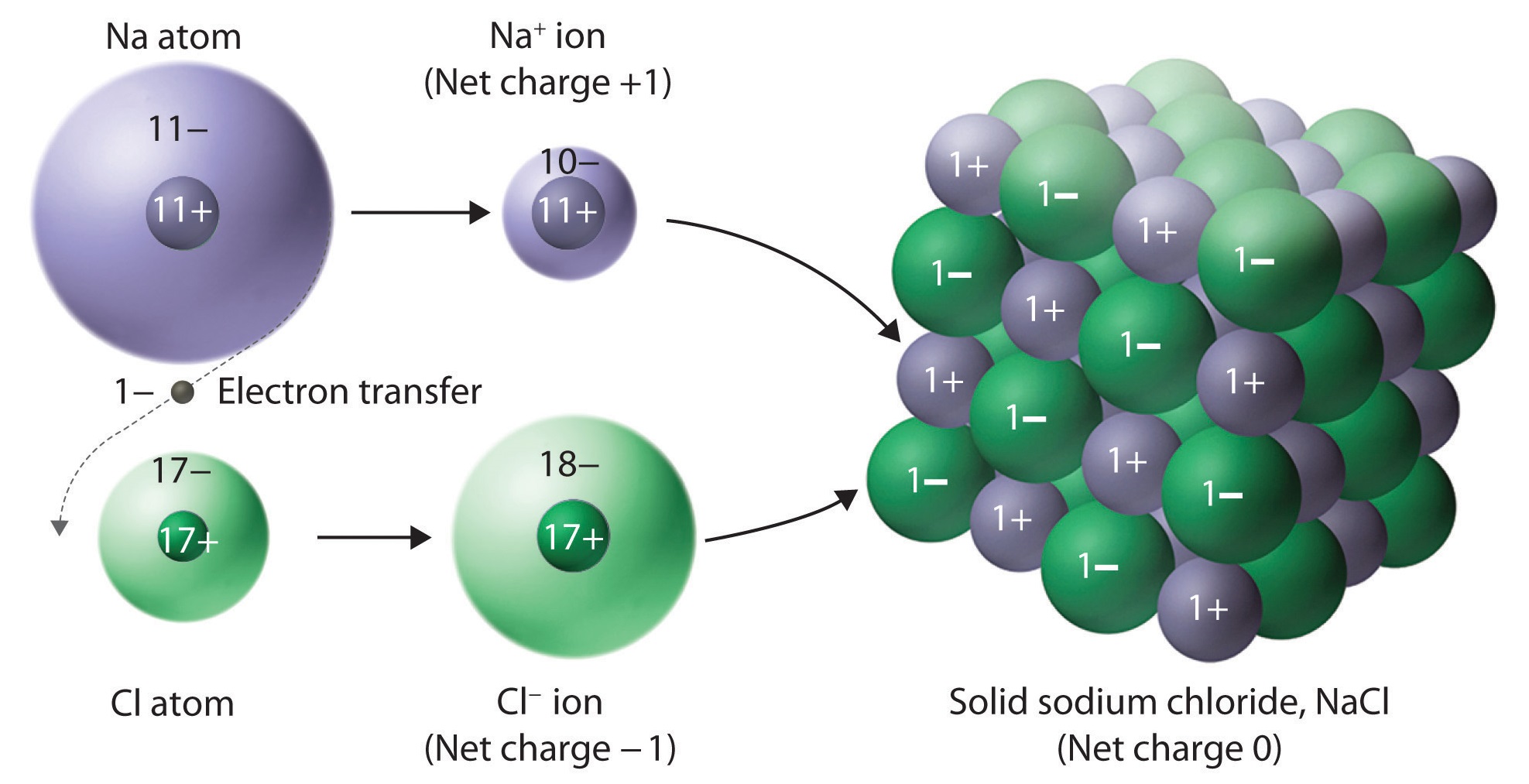
The Formation of Ionic Compounds Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We can think about the formation of such compounds in terms of the periodic properties of the elements
Ionic Bonds Form

Ionic Bonds Form
Ionic bonding Overview. Atoms that have an almost full or almost empty valence shell tend to be very reactive. Strongly Formation. Ionic bonding can result from a redox reaction when atoms of an element (usually metal ), whose ionization Structures. Ionic compounds in the solid state form
Ionic and Covalent Bonds Introduction Ionic bonding is the complete transfer of valence electron s between atoms It is a type of chemical bond that generates two Covalent Bonding Bonding in Organic Chemistry References Problems
4 1 Ionic Bonding Chemistry LibreTexts
8 2 Ionic Bonding Generating Ionic Bonds Ionic bonds form when metals and non metals chemically react By definition a metal is Energetics of Ionic Bond Formation Ionic bonds are formed when positively and negatively charged ions are held together Electron Configuration of Ions How does

Ionic Bond Definition Properties Examples Facts Britannica
The Formation of Ionic Compounds Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl NaCl is a binary ionic compound We can think about the formation of such compounds in terms of the periodic properties of the elements
How Does An Ionic Bond Form Between Sodium And Chlorine Slideshare
/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
Examples Of Ionic Bonds And Compounds
The Ionic Bond Introductory Chemistry
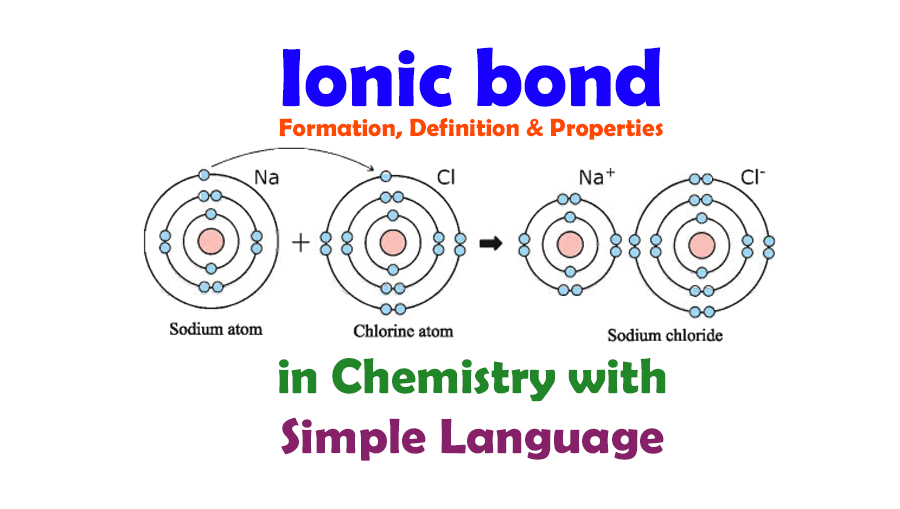
The atom that loses electrons becomes a positively charged ion known as a cation The atom that gains electrons becomes a negatively charged ion known as an anion When the two ions combine via ionic bond they form ionic compounds The types of elements forming ionic bonds are metals and nonmetals 1 4

Ionic Bond Definition Types Properties Examples
Introduction 2 1Early Ideas in Atomic Theory 2 2Evolution of Atomic Theory 2 3Atomic Structure and Symbolism 2 4Chemical Formulas 2 5The Periodic Table 2 6Ionic and Molecular Compounds 2 7Chemical Nomenclature Key Terms Key
Ionic bonds connect a metal and nonmetal element. The bond forms when the atom with less than 4 valence electrons (Sodium) gives its electrons to the atom with more than 4 valence electrons (Chlorine). This forms two ions, Sodium ion with a +1 charge and Chloride ion with a -1 charge. These two ions then form an ionic bond creating Sodium chloride …
Ionic Bonding Wikipedia
4 years ago Two metals can t form an ionic bond The requirements for this bond are the losing of electrons by one element and gaining by another There is no metal in existence that accepts electrons So ionic bond between only metals is not possible 2 comments 41 votes Upvote Downvote Flag

Ionic Bond Definition Types Properties Examples

Ionic Bond Definition Types Properties Examples
Ionic Bonds Form
Introduction 2 1Early Ideas in Atomic Theory 2 2Evolution of Atomic Theory 2 3Atomic Structure and Symbolism 2 4Chemical Formulas 2 5The Periodic Table 2 6Ionic and Molecular Compounds 2 7Chemical Nomenclature Key Terms Key
The Formation of Ionic Compounds Binary ionic compounds are composed of just two elements a metal which forms the cations and a nonmetal which forms the anions For example NaCl is a binary ionic compound We can think about the formation of such compounds in terms of the periodic properties of the elements

Ionic Bonding Wikipedia
20 12 Genetic Engineering Chemistry LibreTexts

Savvy chemist Ionic Bonding 2 Dot And Cross Diagrams Lewis Structures

Periodic Table Ions List Periodic Table Timeline
Ionic Bond And Ionic Bond Formation Definition Properties In Chemistry Tuition Tube
.PNG)