Iso 13485 Software Validation Template - Preview Record of Software Validation template The document is fully editable so that you can adapt it to your company design Documents include placeholder marks for all information you need to complete Each document includes comments and information which guides you through completion
ISO 13485 2016 Section 4 1 6 Quality management system General requirements and 7 5 6 Validation of processes for production and service provision state the following The organisation
Iso 13485 Software Validation Template
Iso 13485 Software Validation Template
Our company is in the process of becoming ISO 13485 compliant and as part of the quality management system, I have to come up with a software validation procedure that explains how we validate software before it goes into production and corresponding testing records.
Templates ISO 13485 Templates Updated January 19 2023 Template SOP Software Validation Sven Piechottka Template Download This is a free template provided by OpenRegulatory
What Are The Software Validation Requirements Of ISO 13485
Long Answer Validation of computer software is specified in section 4 1 6 of ISO 13485 2016 The main messages there are Validate software which is used in the quality management system prior to use and after changes Activities should be proportionate to risk So which software does this include

MDR 2017 745 GSPR Template Easy Medical Device School
This document applies to any software used in device design testing component acceptance manufacturing labelling packaging distribution and complaint handling or to automate any other aspect of a medical device quality system as described in ISO 13485
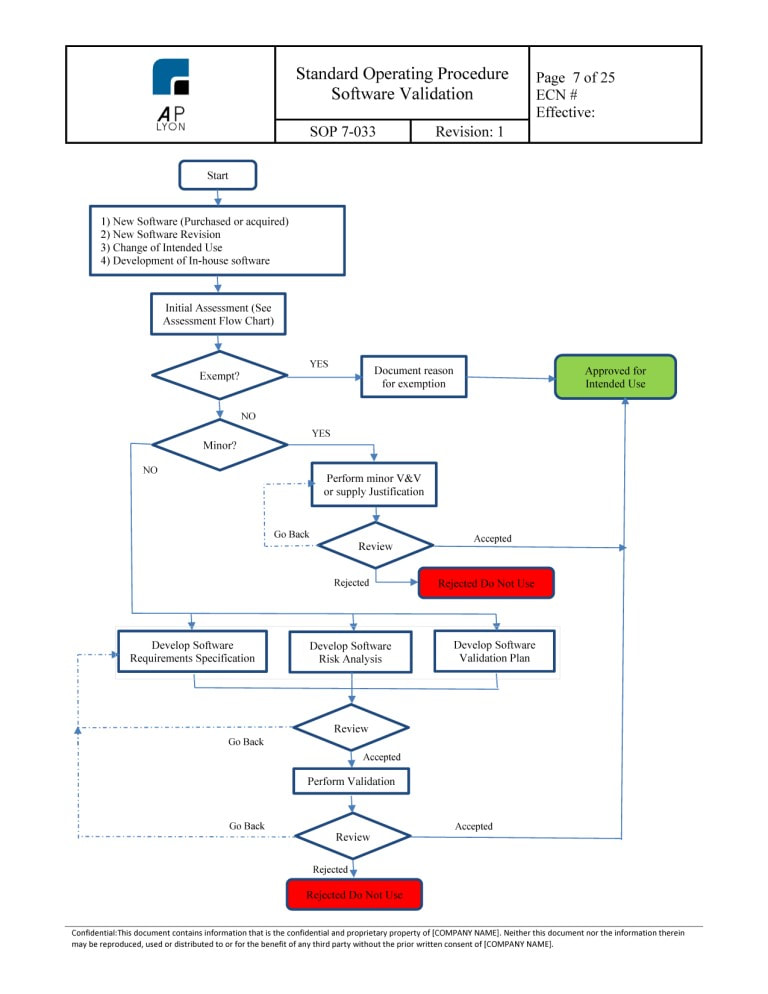
Software Validation Procedure Iso 13485 Template
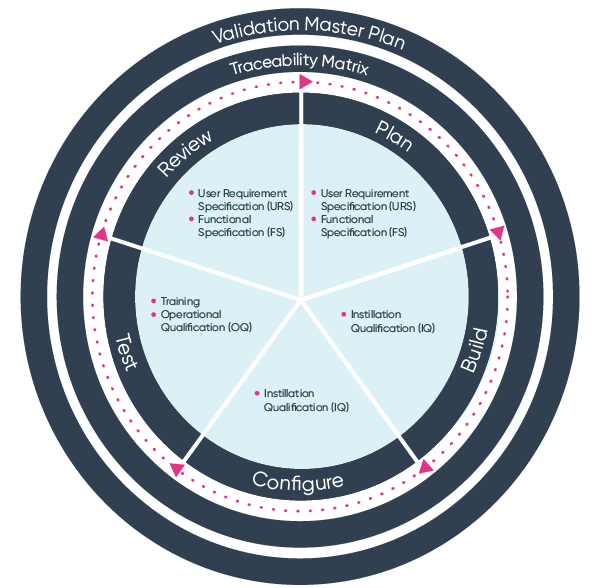
ISO 13485 Software Validation Process Ideagen
Record Of Software Validation ISO 13485 Templates Advisera
The ISO 13485 is the standard for quality management in the medical device industry Here are all our posts on this standard and also all questions our consulting clients have asked us about the ISO 13485 so far Additionally we publish all our document templates for the ISO 13485 for free so scroll down and have a look at those
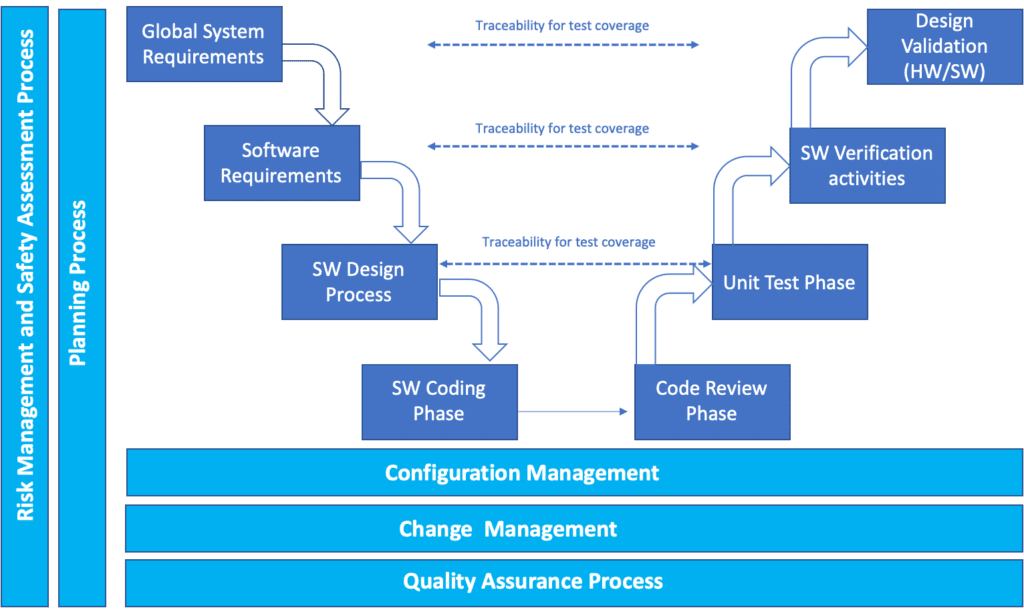
Software Design For Medical Devices
The procedure should reference ISO 13485 2016 and outline a risk based approach to evaluating current updated and new software that will be used in the quality system This procedure will help guide your company to properly evaluating all QMS software throughout its lifecycle
SYS-044 - Software Development and Validation Procedure. SYS-044 Software Development and Validation Procedure; This procedure is intended to meet the requirements of ISO 13485:2016, Clause 7.3.6 and 7.3.7 for design verification and design validation of medical device products.
Software Validation Template Policies Amp Procedures
Free ISO 13485 Software Validation Template You can buy the ISO 13485 standard here email us here from your work email verifiable domain from company website to receive a FREE copy of this SOP free of charge
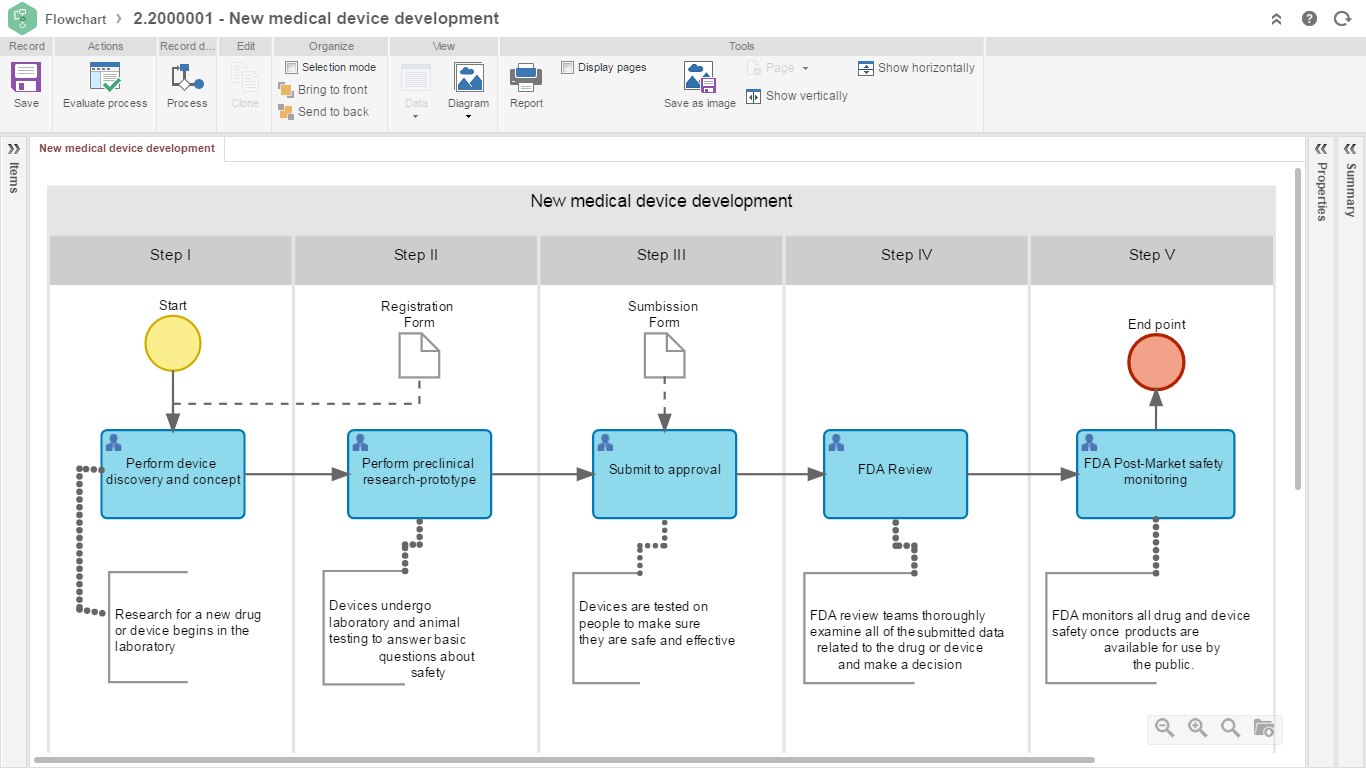
ISO 13485 SoftExpert Software
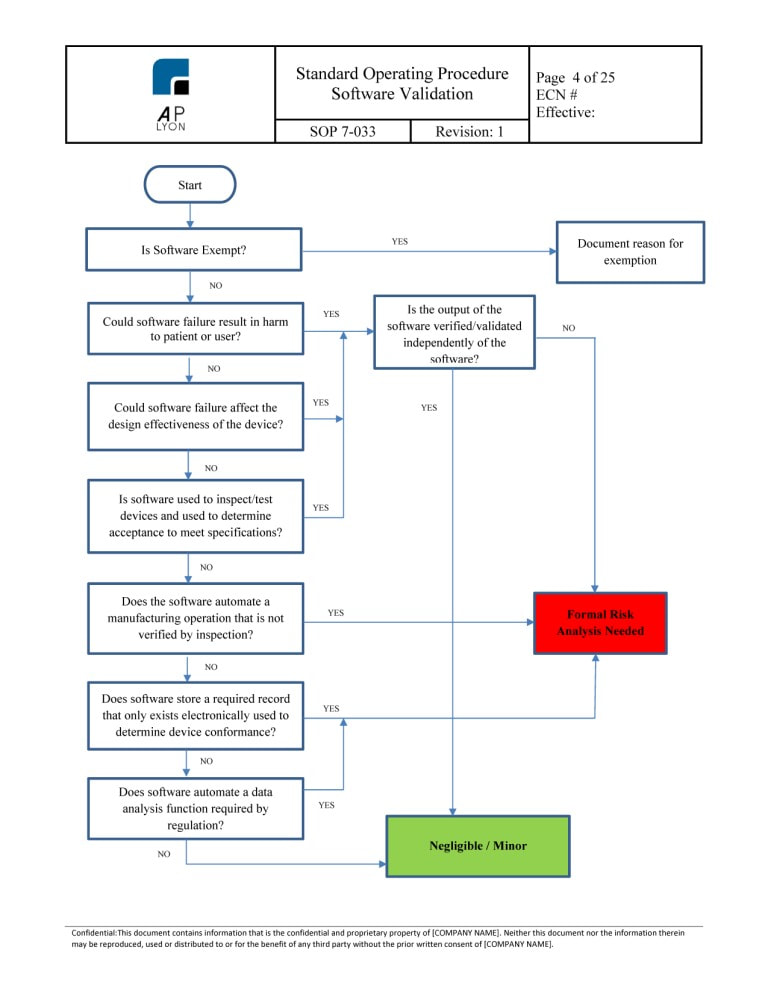
Iso 13485 Test Method Validation Headsultra
Iso 13485 Software Validation Template
The procedure should reference ISO 13485 2016 and outline a risk based approach to evaluating current updated and new software that will be used in the quality system This procedure will help guide your company to properly evaluating all QMS software throughout its lifecycle
ISO 13485 2016 Section 4 1 6 Quality management system General requirements and 7 5 6 Validation of processes for production and service provision state the following The organisation
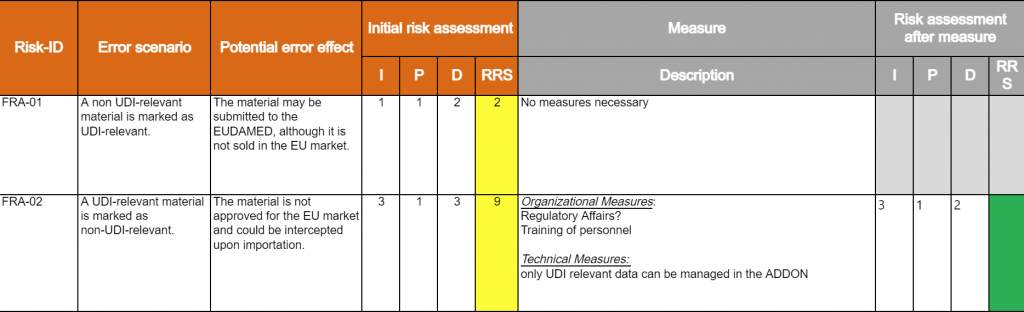
Computer System Validation CSV Gem ss ISO 13485 Europe IT

Iso 13485 2016 Pdf Sdbilla
Iso 13485 21 CFR 820 Template Documentation Operational Procedure Qop

Iso 13485 Software Validation Template PDF Template

Software Validation Template Iso 13485
