Medical Device Traceability Matrix Template - The Requirements Traceability Matrix is used to track the project requirements through the Project Life Cycle It documents each requirement the source of the requirement and traces how the requirement will be addressed through the project deliverables stakeholdermap
The first step to build a requirements traceability matrix is to create the template or shell of your matrix This is where you ll determine what you want to trace and why and collect the necessary documents 1 Define Your Goal Your first step when creating a traceability matrix whether you re using Excel or a dedicated requirements
Medical Device Traceability Matrix Template

Medical Device Traceability Matrix Template
The following elements define a traceability matrix for Software as a Medical Device (SaMD): Goal/User Need : The user needs a SaMD that provides accurate diagnosis for a specific medical condition. Design Input : The SaMD must achieve a diagnostic accuracy of at least 95% in clinical trials.
1 Hello All Our Notified Body has requested we provide a traceability matrix with detailed verbiage of indications statement contraindications warnings and precautions and complications showing traceability across IFUs Risk Assessments and Clinical Evaluation Report
How To Create A Traceability Matrix Template And Samples
What is a traceability matrix A traceability matrix is a visual representation of the relationships and linkages between key areas of your design process for example your User Needs Design Inputs Design Outputs Design Verification and Design Validation

FDA Expectations For Traceability In Device Diagnostic Design
A library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them Charting Success in 2024 Participate in Our Annual Industry Benchmark Survey CareersSupportContact Sales Products Quality Clinical Academy

Medical Device Risk Assessment Template Generatordast
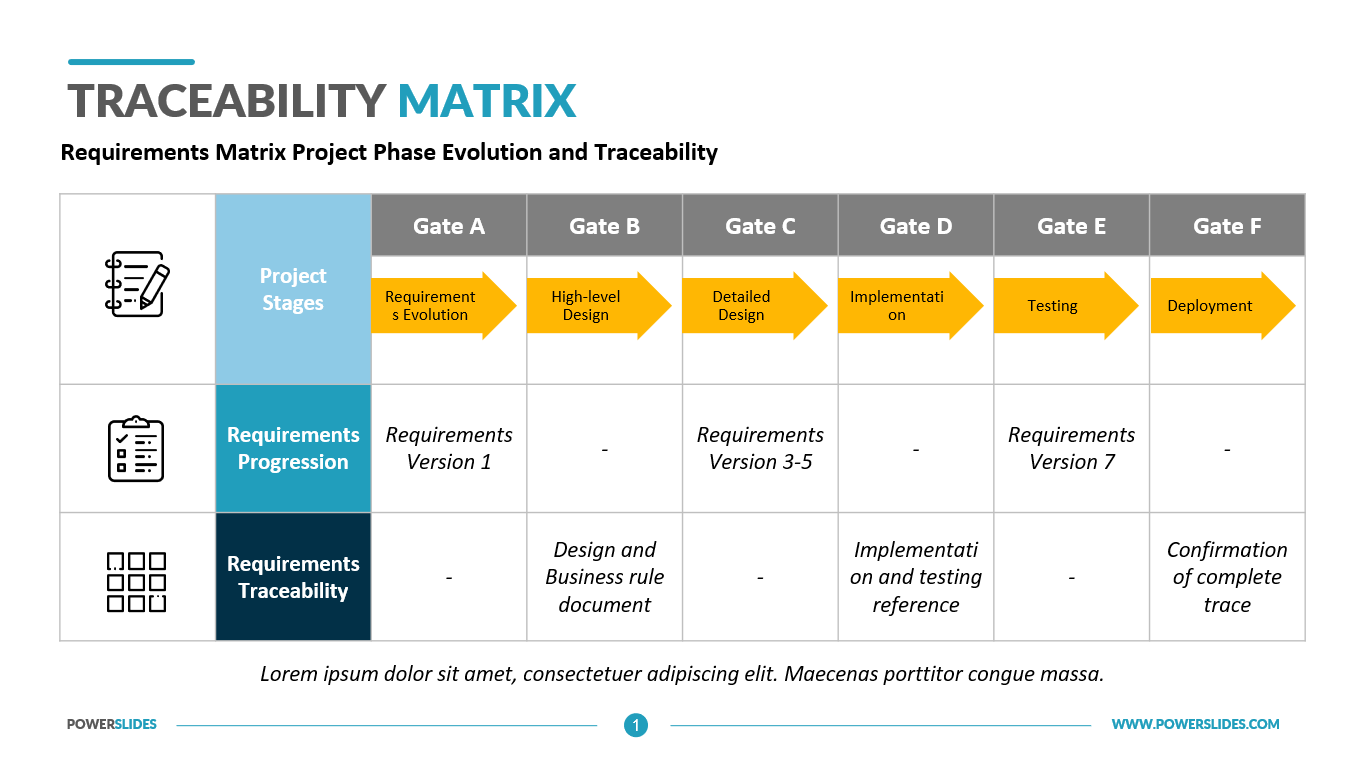
The Benefits Of Using A Requirement Traceability Matrix In Software
Requirements Traceability Matrix Excel Template FREE
With the right tool a traceability matrix can significantly reduce your project risk set the foundation of your product lifecycle and give you full control over your project qmsWrapper lets you build a Traceability Matrix for any of your products or even a medical device and its regulatory needs
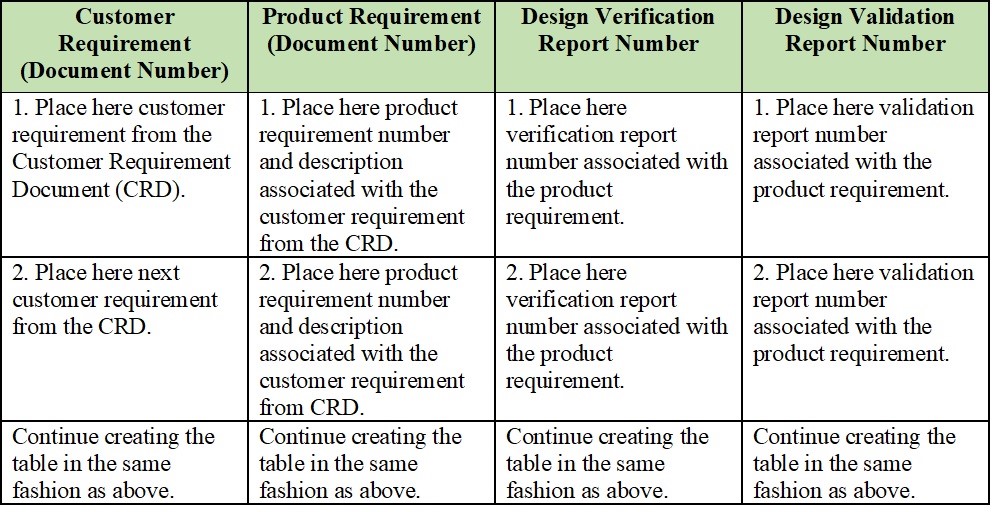
Medical Device Test Method Validation Template
FREE TEMPLATE Traceability Matrix This traceability matrix is an essential component of your Design History File DHF It shows the linkages between User Needs UNs Design Inputs DIs verification and validation Download this template customize with your own branding and get started in assembling this critical part of your DHF
A traceability matrix is an invaluable tool to show a high-level view and the flow of medical device product development from beginning to end. Best practice product developers have relied on Design Controls traceability for many, many years.
A Guide To FDA S Medical Device Traceability Matrix Ketryx
Risk Management Plan Template Medical Device and ISO 14971 Risk Analysis Hazard Traceability Matrix Design and Development Plan Template Medical Device per ISO 13485 and 21 CFR 820 Status Report Checklist ISO 14971 2007 to ISO 14971 2019 Risk Management Plan Template Project Charter Templates
Defect Traceability Matrix
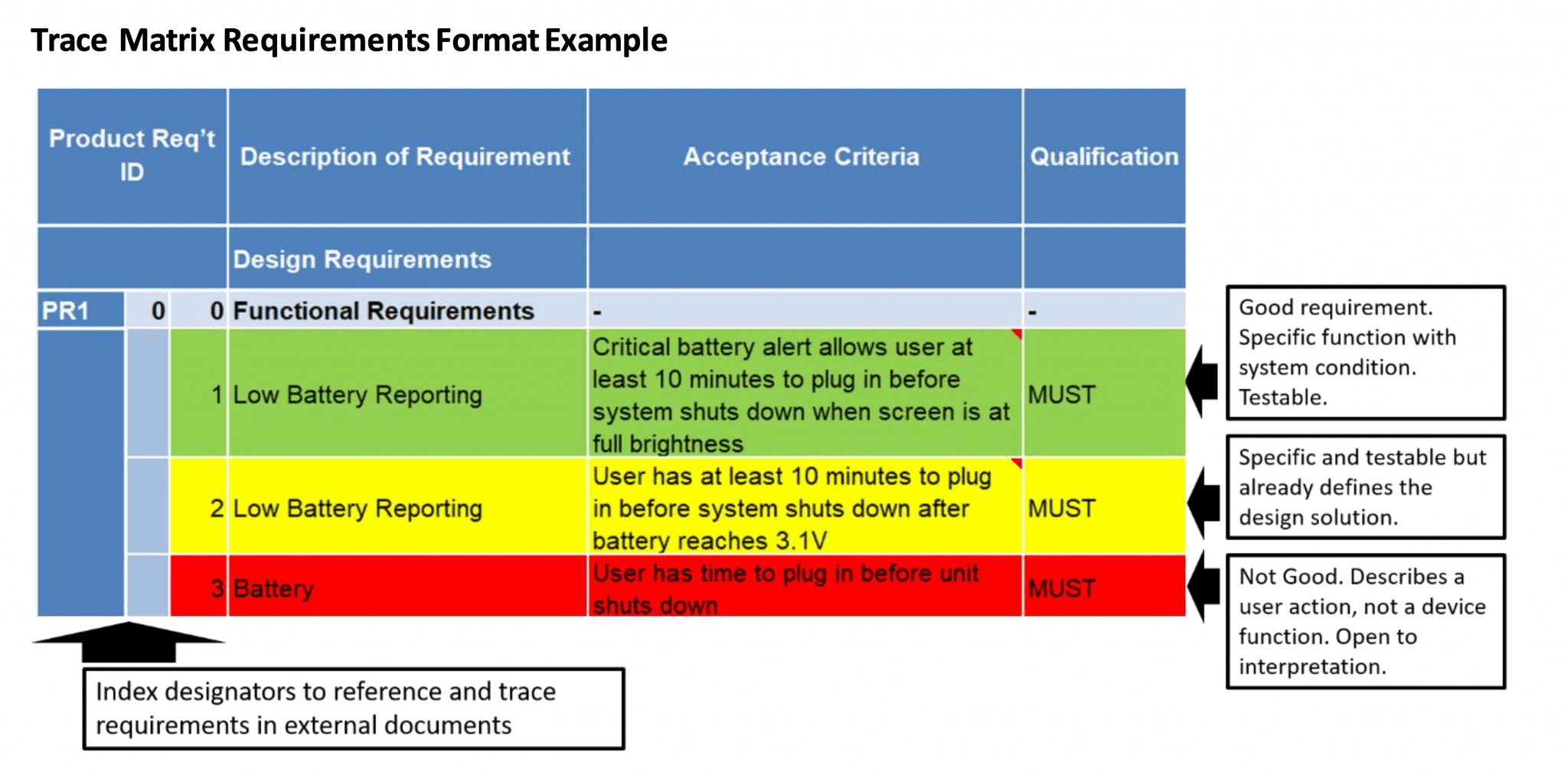
How To Define Product Requirements For Medical Devices Simbex
Medical Device Traceability Matrix Template
FREE TEMPLATE Traceability Matrix This traceability matrix is an essential component of your Design History File DHF It shows the linkages between User Needs UNs Design Inputs DIs verification and validation Download this template customize with your own branding and get started in assembling this critical part of your DHF
The first step to build a requirements traceability matrix is to create the template or shell of your matrix This is where you ll determine what you want to trace and why and collect the necessary documents 1 Define Your Goal Your first step when creating a traceability matrix whether you re using Excel or a dedicated requirements
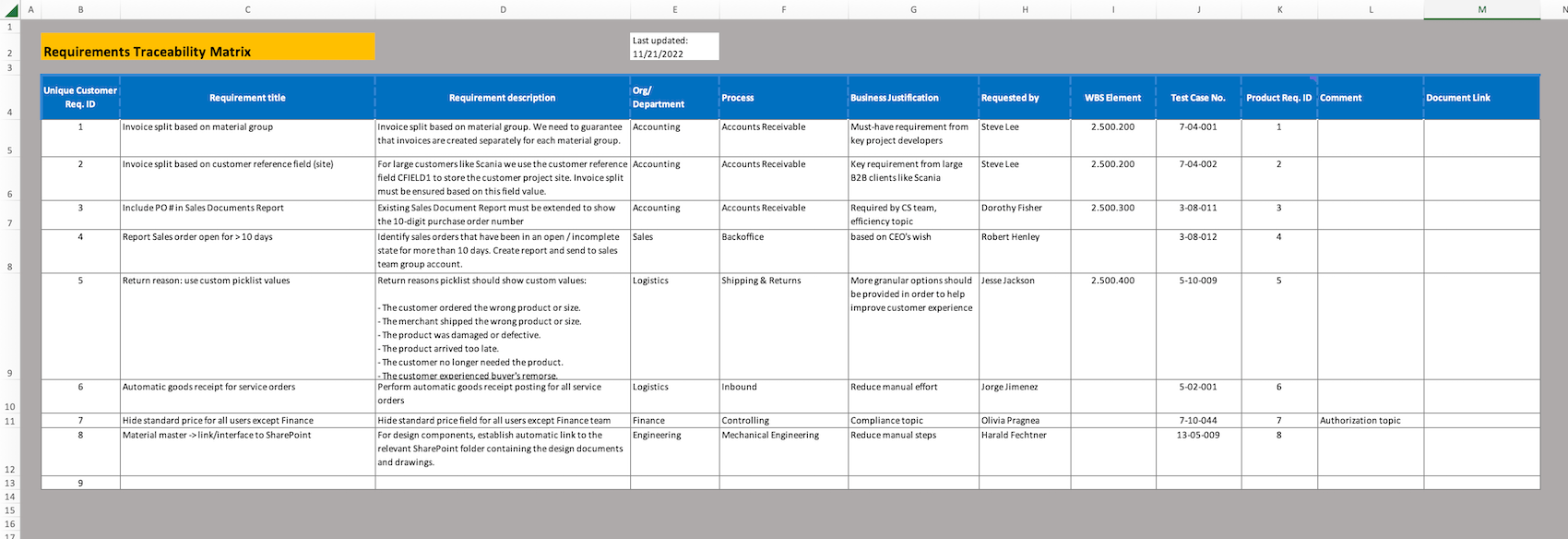
My Requirements Traceability Matrix Template Excel

Project Management Templates Software Projects Gantt Chart Templates
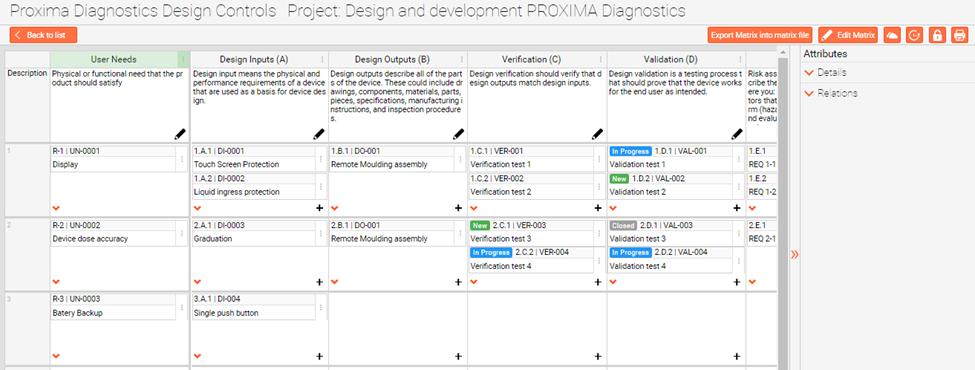
Design Controls In The Traceability Matrix QmsWrapper

L14 05 Requirements Traceability Matrix Example YouTube

Design Traceability Matrix Download Scientific Diagram
