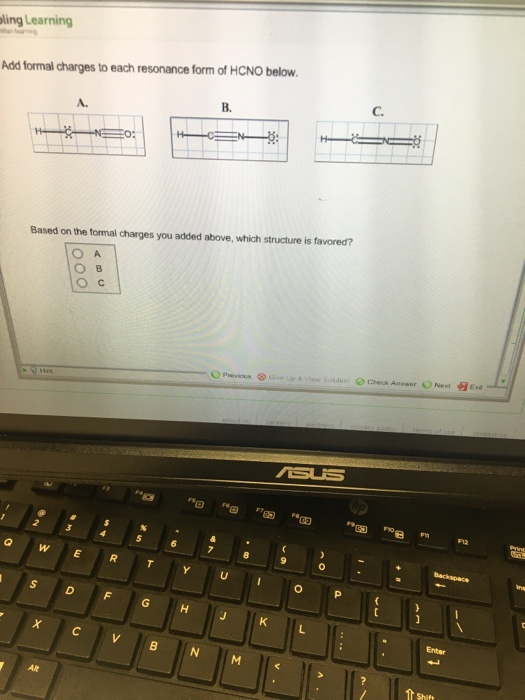
Add Formal Charges To Each Resonance Form Of Hcno. - 1 A 2 B 3 C Assigning formal charge The formal charge of each atom in a Lewis dot structure is 1 2 the number of electrons in its bonds the total of its electrons in lone pairs
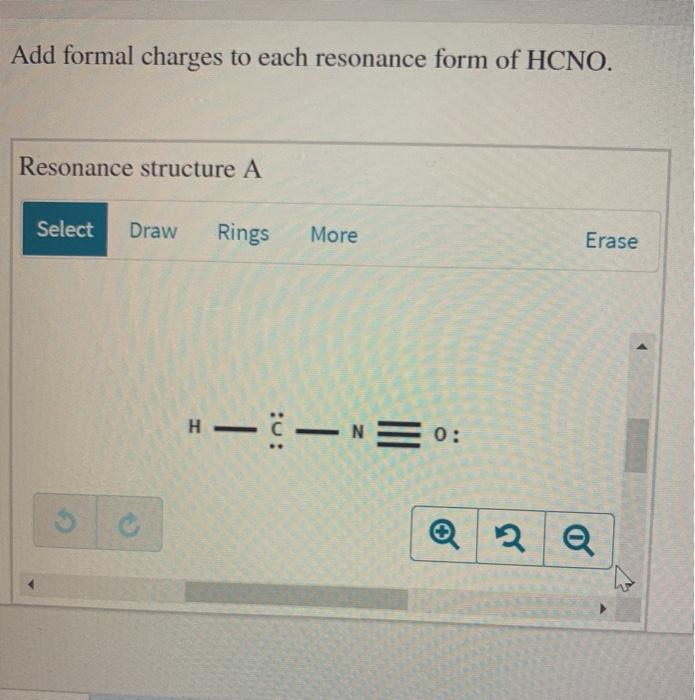
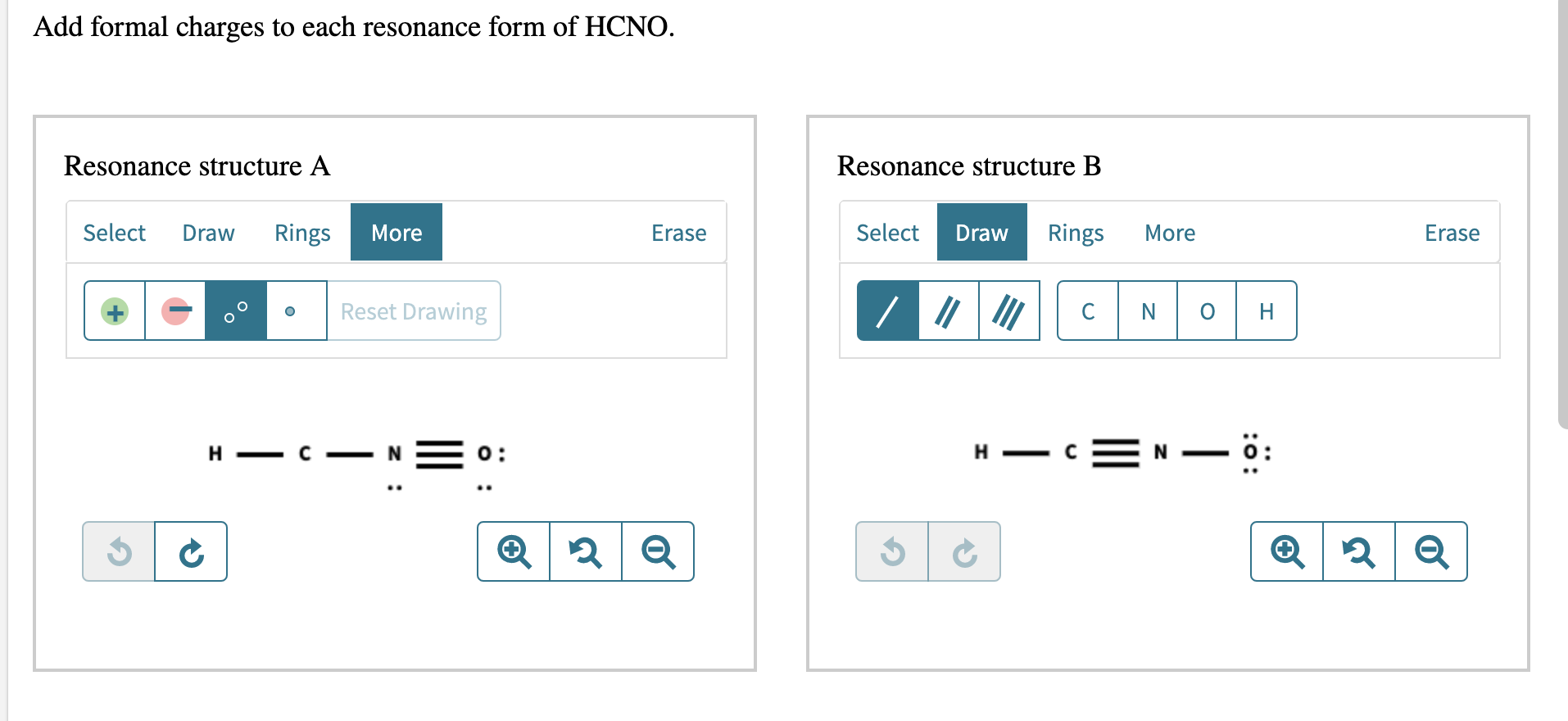
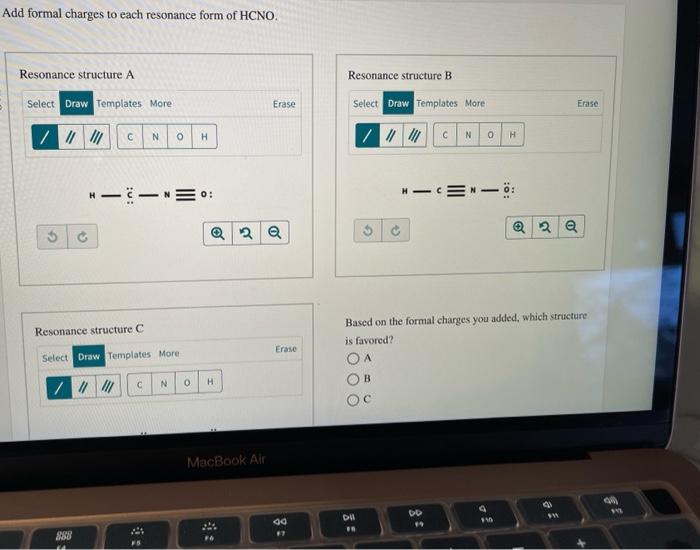
Add formal charges to each resonance form of HCNO Resonance structure A Resonance structure B Select Rings Draw More Erase Select Draw Rings More C N H C N C O H Based on the formal charges you added which struct Resonance structure C is favored Erase More Rings Select Draw O A N O c H z This problem has been solved
Add Formal Charges To Each Resonance Form Of Hcno.
Add Formal Charges To Each Resonance Form Of Hcno.
1. Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons. 2. Draw the bond connectivities: The three oxygens are drawn in the shape of a triangle with the nitrogen at the center of the triangle. 3. Add octet electrons to the atoms bonded to the center atom: 4.
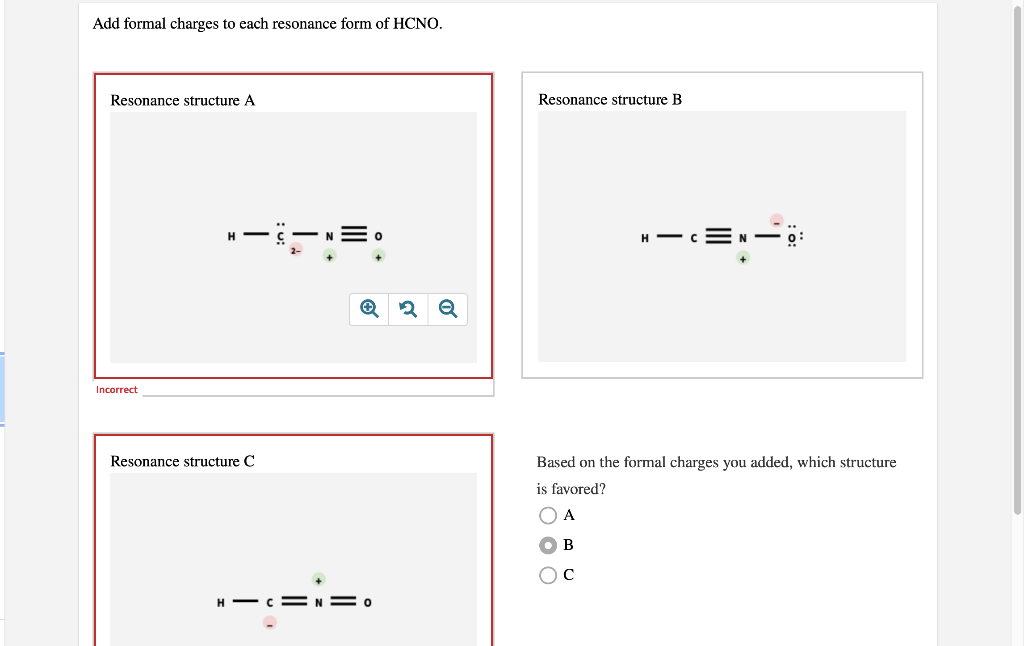
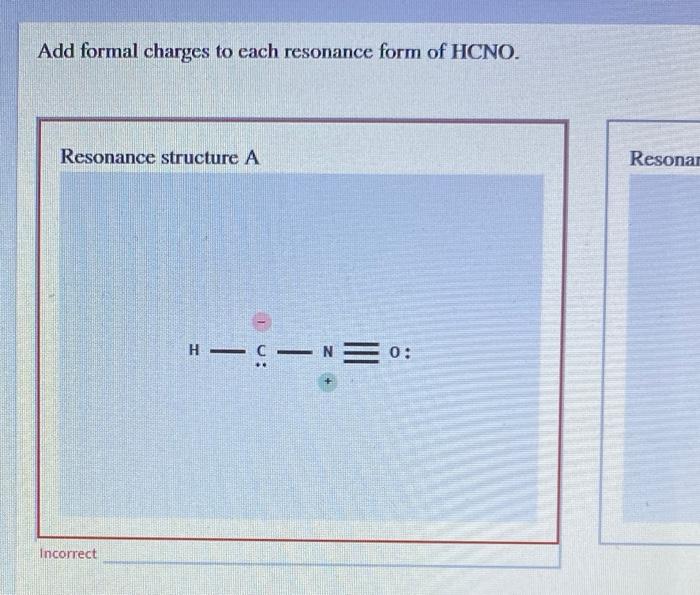
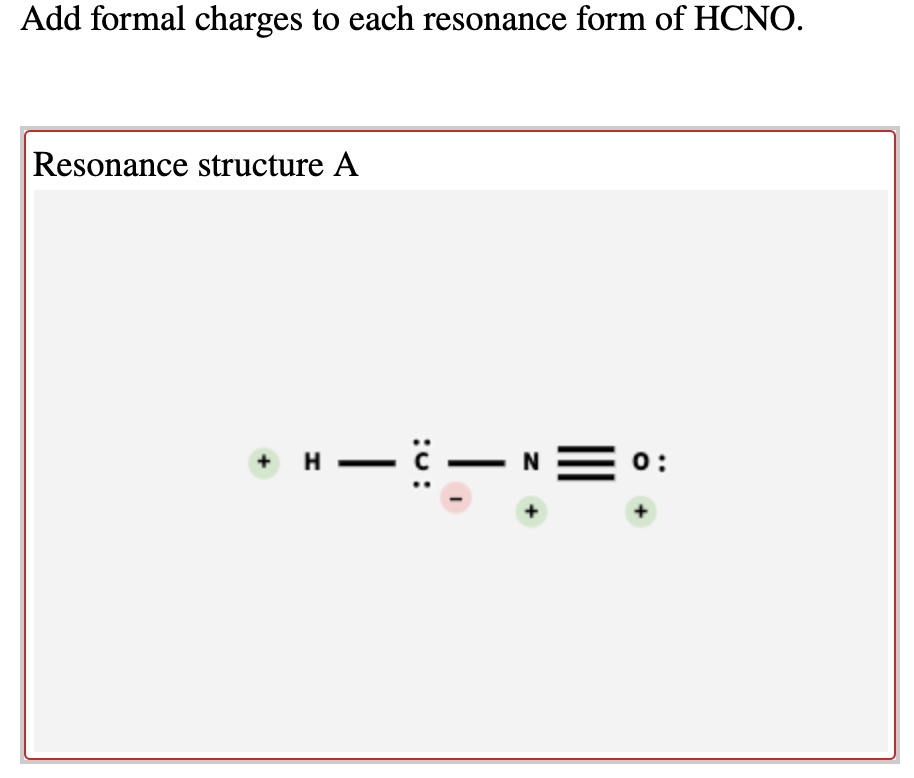
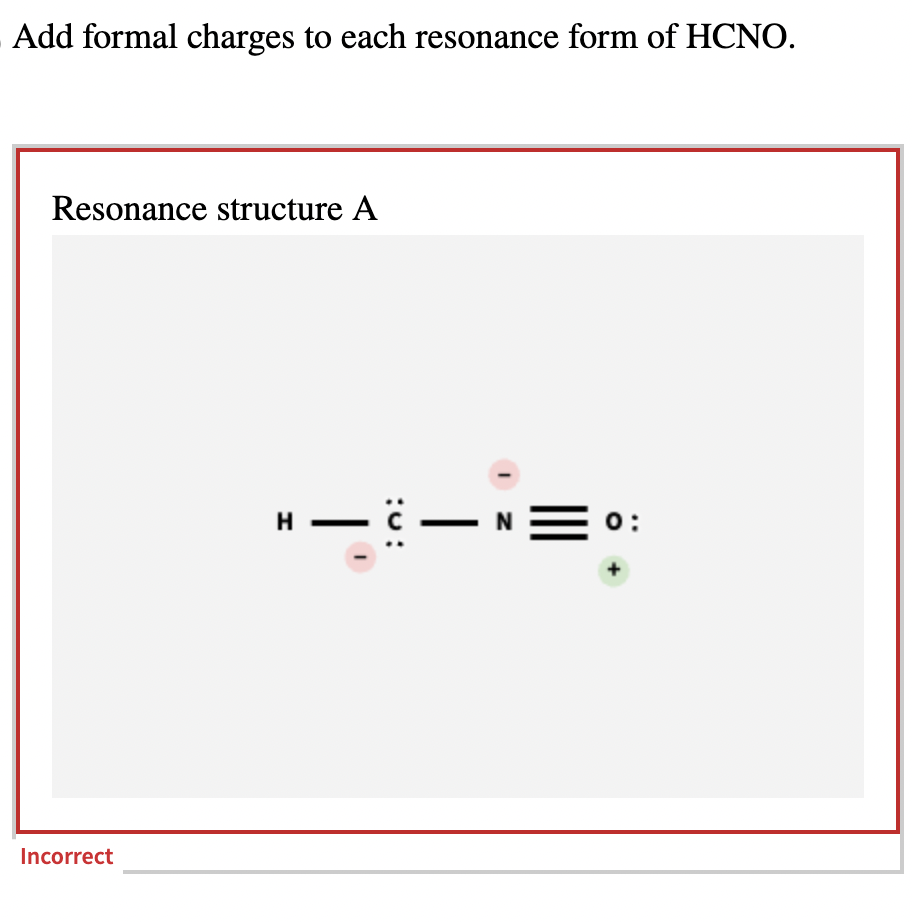
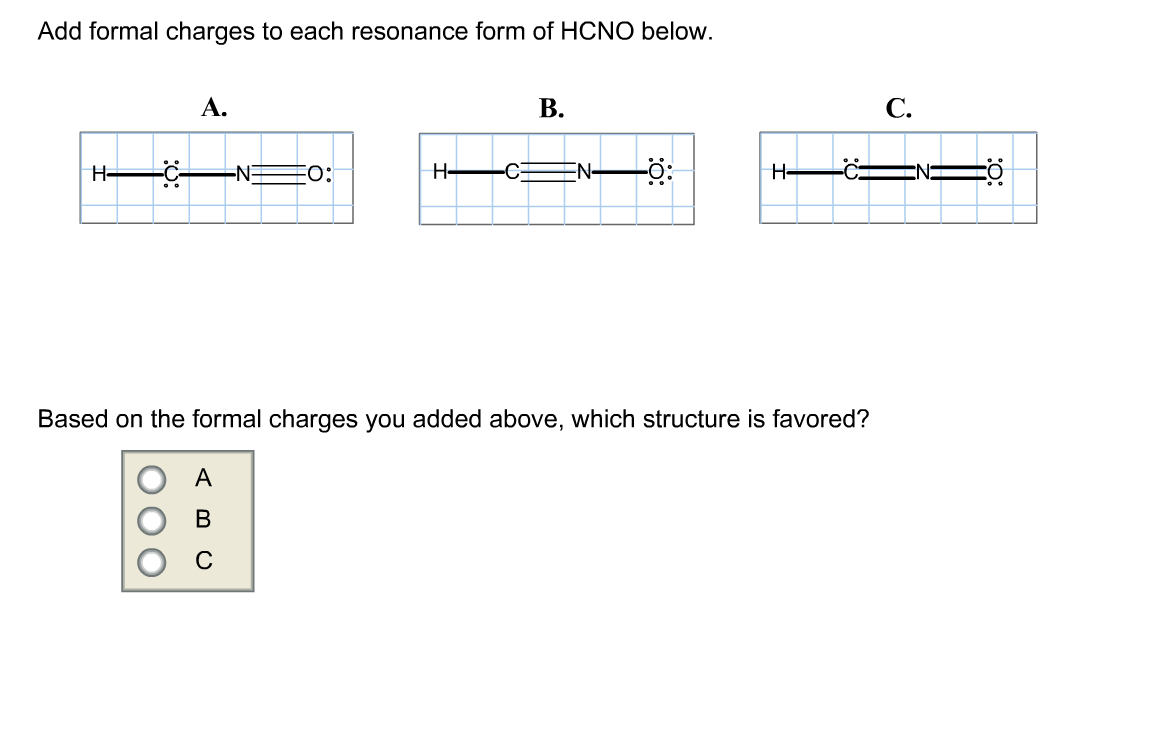
Add formal charges to each resonance form of HCNO Based on the formal charges you added which structure is favored A triple bond between N and O single bonds between all other atoms 2 on C 1 on N 1 on O B triple bond between C and N single bonds between all other atoms 1 on N 1 on O C double bonds between C N and O
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
This question asks to add formal charges to each resonance form of HCNO The first resonance structure has a FC of 2 on C 1 on N and 1 on O The second has a FC of 1 on N and 1 on O The third structure has a FC of 1 on N and 1 on C The question then asks which structure is favored based on these formal charges
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Each Cl atom now has seven electrons assigned to it and the I atom has eight Subtract this number from the number of valence electrons for the neutral atom I 7 8 1 Cl 7 7 0 The sum of the formal charges of all the atoms equals 1 which is identical to the charge of the ion 1 Exercise 6 5 1
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Add Formal Charges To Each Resonance Form Of HCNO Below Based On The
Step 1 2 First we need to draw the Lewis structure of HCNO H C N O Answer Next we need to determine the formal charges for each atom in the molecule
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
The sum of the formal charges of all the atoms in a neutral molecule equals zero The sum of the formal charges of all the atoms in an ion equals the charge of the ion Uses of Formal Charges Formal charges can help identify the more important resonance structures that is hitherto we have treated all resonance structures as equal but this
Add formal charges to each resonance form of HCNO below: You need to put formal charge on each atom in these structures. To do so, click on structure to open the toolbar; click the button labeled + (equals 3) click on an atom. Type the desired charge. Based on the formal charges you added above, which structure is favored?
8 3 Resonance Structures And Formal Charge Chemistry LibreTexts
The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here Note that the sum of the formal charges in each case is equal to the charge of the ion 1
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Add Formal Charges To Each Resonance Form Of Hcno.
The sum of the formal charges of all the atoms in a neutral molecule equals zero The sum of the formal charges of all the atoms in an ion equals the charge of the ion Uses of Formal Charges Formal charges can help identify the more important resonance structures that is hitherto we have treated all resonance structures as equal but this
Add formal charges to each resonance form of HCNO Resonance structure A Resonance structure B Select Rings Draw More Erase Select Draw Rings More C N H C N C O H Based on the formal charges you added which struct Resonance structure C is favored Erase More Rings Select Draw O A N O c H z This problem has been solved
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg

Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg
Solved Add Formal Charges To Each Resonance Form Of HCNO Chegg

Add Formal Charges To Each Resonance Form Of HCNO Below WizEdu