Choose The Kinetic Product Formed During The Reaction Depicted Below. - The kinetic product benefits from the colder temperature because it has a lower activation energy and the activation energy of the reverse reaction products reforming the reactants is to great for the reverse reaction to proceed Once the kinetic reaction occurs which is the faster of the two reactions the kinetic products won t be able to
The general rate law for a unimolecular elementary reaction A products is rate k A r a t e k A For bimolecular reactions the reaction rate depends on the number of collisions per unit time which is proportional to the product of the concentrations of the reactants as shown in Figur e 14 6 1 14 6 1
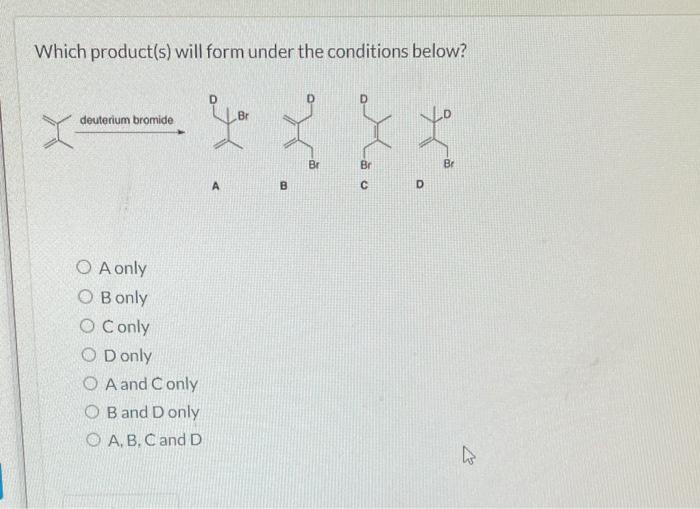
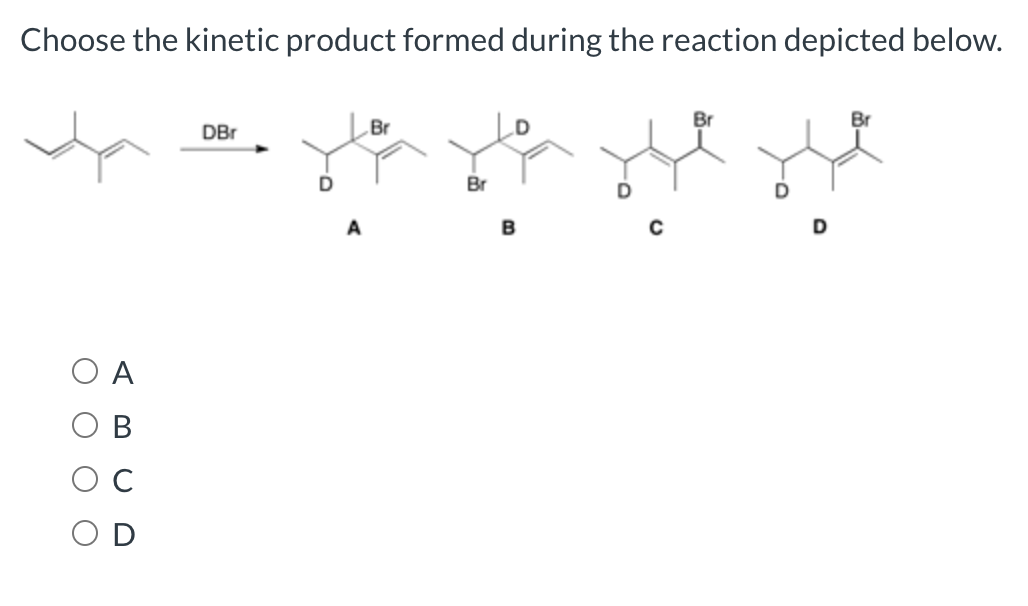
Choose The Kinetic Product Formed During The Reaction Depicted Below.
Choose The Kinetic Product Formed During The Reaction Depicted Below.
The reaction product which forms with a higher rate of reaction is called the Kinetic Product and when the kinetic product dominates, the reaction is said to be under Kinetic Control. At higher temperatures the reaction to form both products becomes reversible and a reaction equilibrium is reached. When a reaction is reversible the major ...
Science Chemistry Chemistry questions and answers Choose the kinetic product formed during the reaction depicted below This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer Question Choose the kinetic product formed during the reaction depicted below
14 6 Reaction Mechanisms Chemistry LibreTexts
Choose the kinetic product formed during the reaction depicted below A B C D This question hasn t been solved yet Ask an expert Question Choose the kinetic product formed during the reaction depicted below A B C D Show transcribed image text Expert Answer Transcribed image text
Solved Choose The Kinetic Product Formed During The Reaction Chegg
In other words reactions that release energy have a G 0 A negative G also means that the products of the reaction have less free energy than the reactants because they gave off some free energy during the reaction Reactions that have a negative G and consequently release free energy are called exergonic reactions
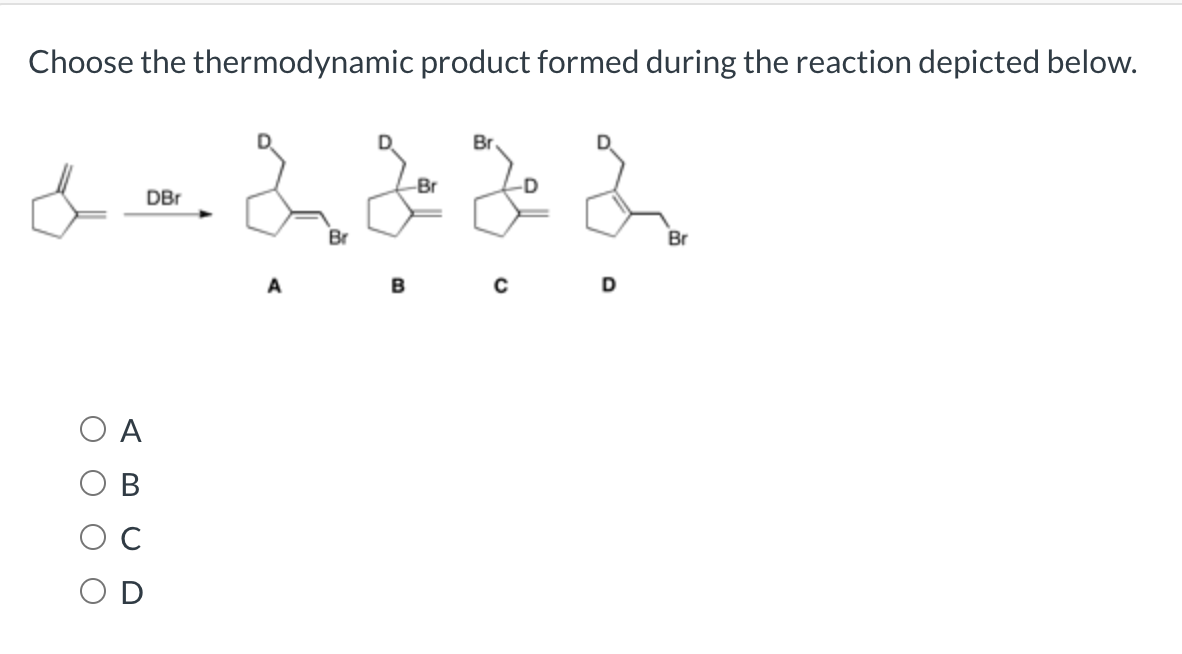
Solved Choose The Thermodynamic Product Formed During The Reaction Course Hero
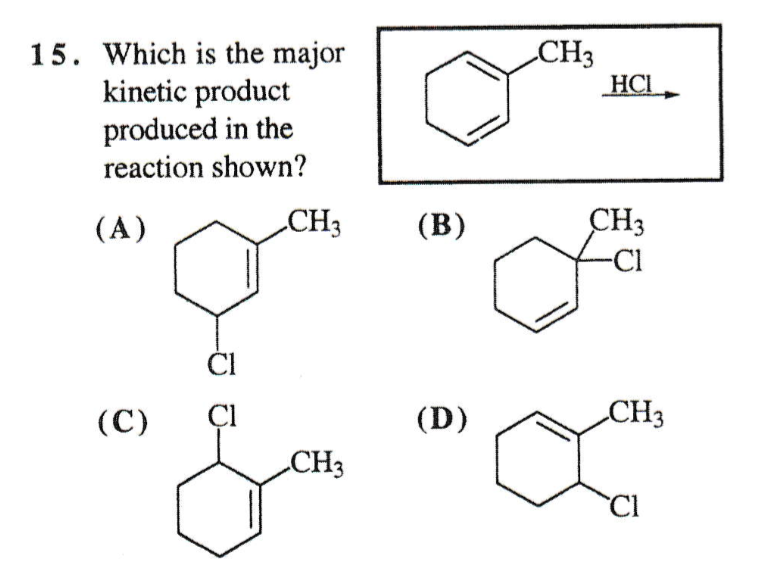
Solved Which Is The Major Kinetic Product Produced In The Chegg
Kinetic And Thermodynamic Enolates Video Khan Academy
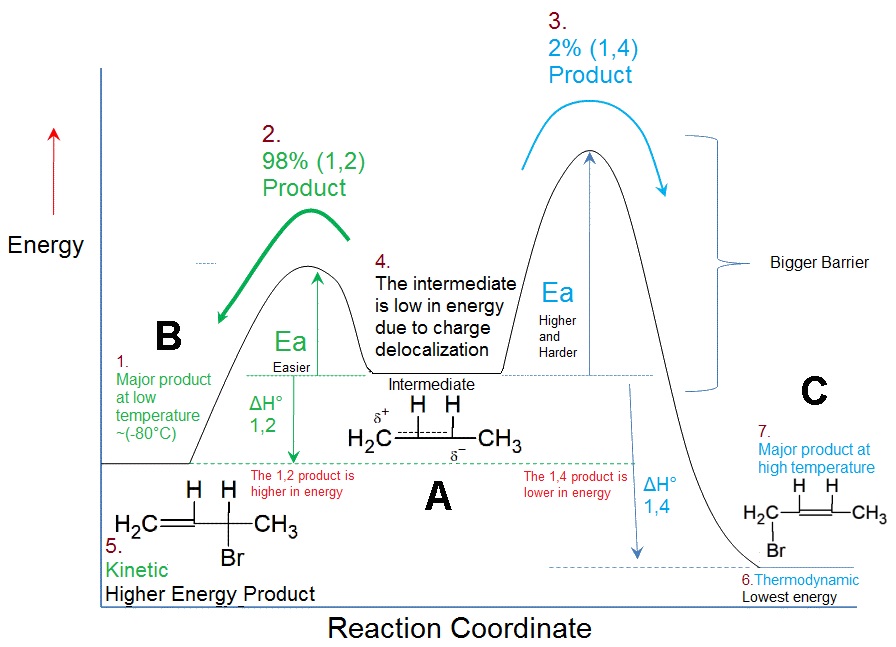
14 3 Kinetic versus Thermodynamic Control of Reactions Electrophilic addition to a conjugated diene at or below room temperature normally leads to a mixture of products in which the 1 2 adduct predominates over the 1 4 adduct When the same reaction is carried out at higher temperatures however the product ratio often changes and the 1 4
Solved Choose The Thermodynamic Product Formed During The Chegg
Chapter 14 Chemical Equilibrium Unit 5 Kinetics and Equilibria
Chemistry 350: Organic Chemistry I 14: Conjugated Compounds and Ultraviolet Spectroscopy
16 9 Kinetic Versus Thermodynamic Products Chemistry LibreTexts
1 Answer Sorted by 1 A product formed under kinetic conditions may or not be reversible under the conditions of the reaction Here is an example of one that is reversible under the reaction conditions Kinetic control is determined by the difference in activation energy E a of the two pathways

The Kinetically Controlled Product Is Formed Faster Than The Thermodynamically Controlled
Solved Question 17 What Is The Kinetic Product Of The Following Reaction Course Hero
Choose The Kinetic Product Formed During The Reaction Depicted Below.
Chapter 14 Chemical Equilibrium Unit 5 Kinetics and Equilibria
The general rate law for a unimolecular elementary reaction A products is rate k A r a t e k A For bimolecular reactions the reaction rate depends on the number of collisions per unit time which is proportional to the product of the concentrations of the reactants as shown in Figur e 14 6 1 14 6 1

2 For The Sw2 Reaction Depicted Below a Please Form All Possible Products And b Draw
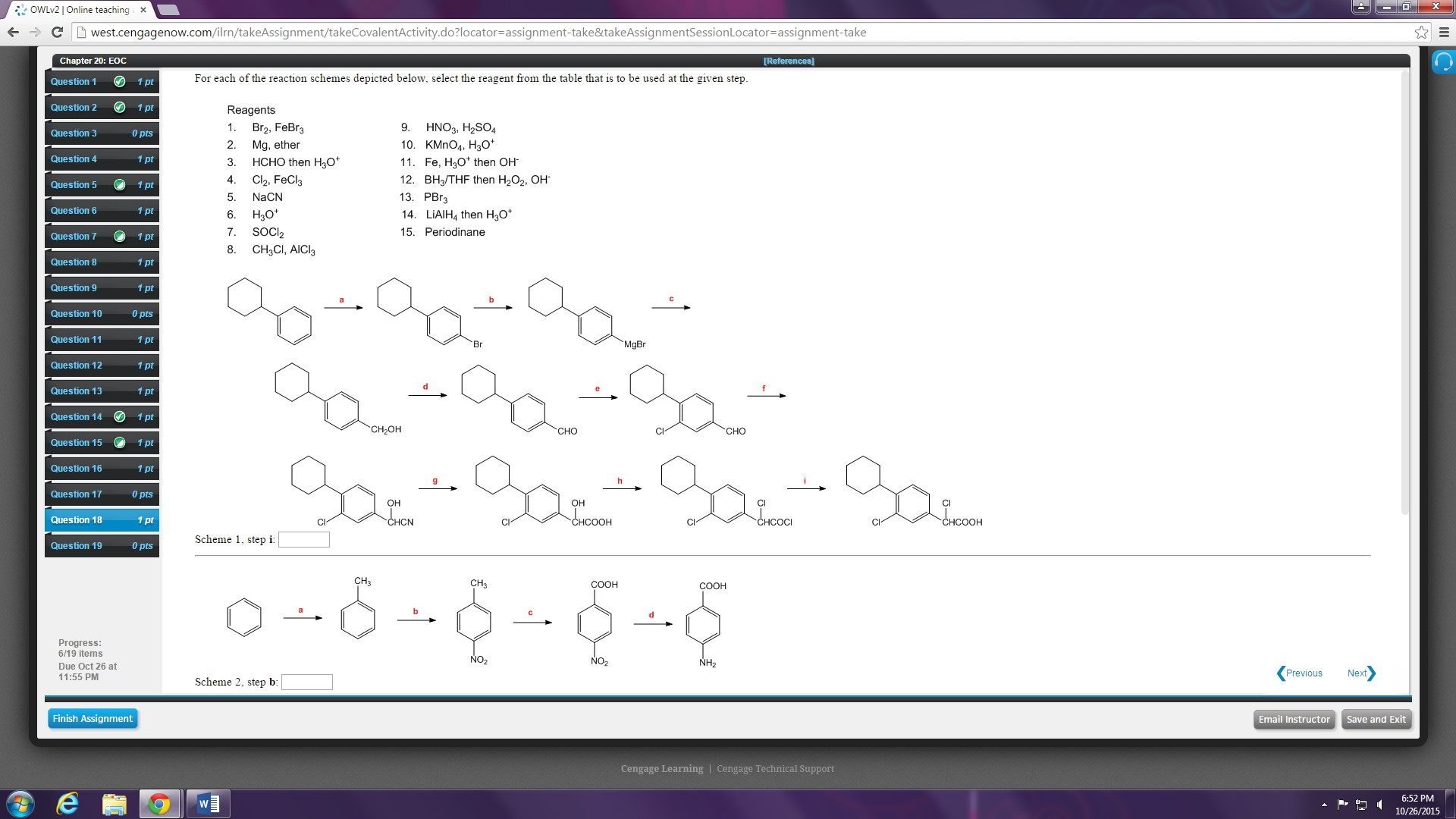
Solved For Each Of The Reaction Schemes Depicted Below Chegg
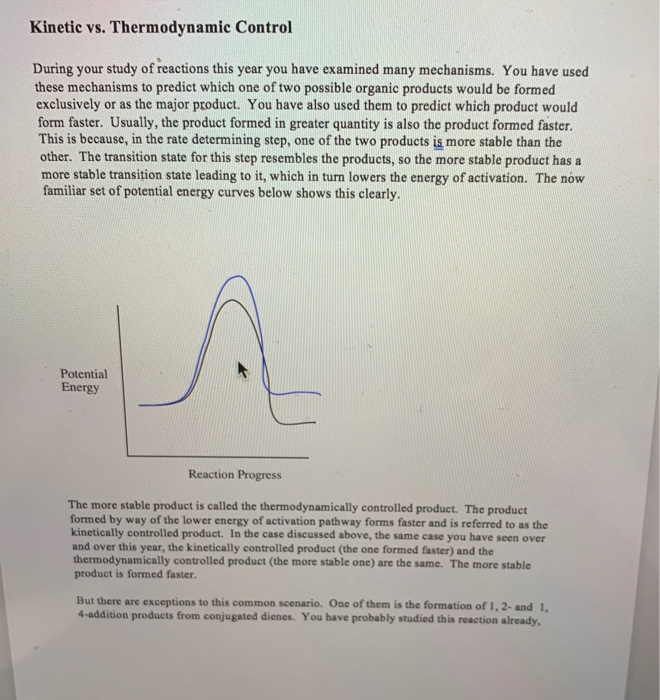
Solved Kinetic Vs Thermodynamic Control During Your Study Chegg
Solved Part A Predict The Major Organic Product Formed When Chegg
Draw The Kinetic Product Of The Following Reaction The Expert