Gold Forms A Substitutional Solid Solution With Silver - Gold forms a substitutional solid solution with silver Compute the number of gold atoms per cubic centimeter for a silver gold alloy that contains 10 wt Au and 90 wt Ag The densities of
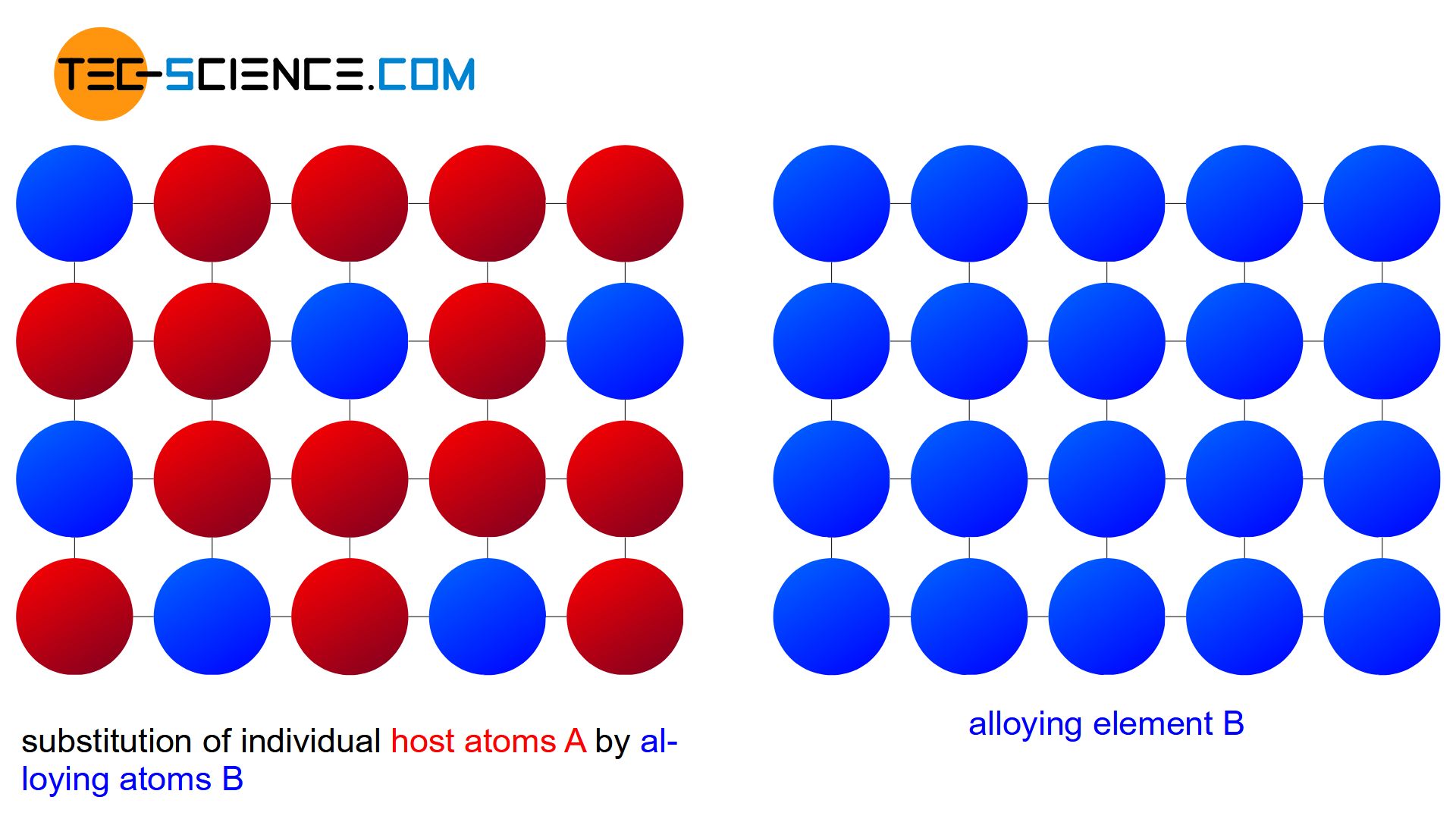
Substitutional solid solutions are formed by melting elements into liquid form mixing them together then allowing the mixture to cool and solidify Elements will form substitutional
Gold Forms A Substitutional Solid Solution With Silver

Gold Forms A Substitutional Solid Solution With Silver
Find step-by-step Engineering solutions and your answer to the following textbook question: Gold forms a substitutional solid solution with silver. Compute the weight percent of gold that must be added to silver to yield an alloy that contains $5.5 \times 10^21 \mathrmAu$ atoms per cubic centimeter. The densities of pure $\mathrmAu$ …
Gold forms a substitutional solid solution with silver Calculate the number of gold atoms per cubic centimeter in atoms cm3 for a silver gold alloy that contains 19 0 wt Au and 81 0 wt Ag The densities of pure gold and silver are 19 32 and 10 49 g cm3 respectively and their respective atomic weights are 196 97 and 107 87 g mol
Substitutional Solid Solution Overview Rules Amp Examples
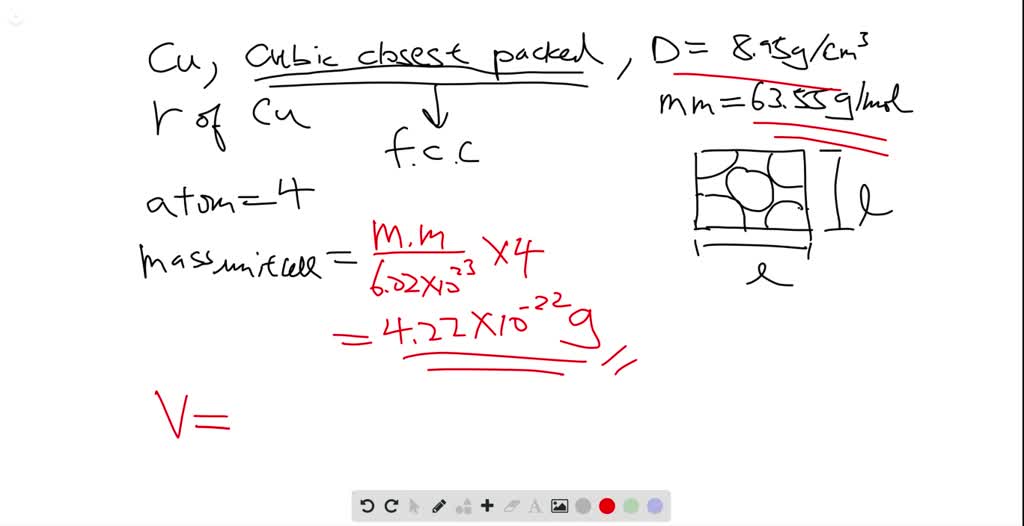
Gold forms a substitutional solid solution with silver Compute the number of gold atoms per cubic centimeter for a silver gold alloy that contains 10 wt Au and 90 wt Ag The densities of pure gold and silver are 19 32 and 10 49 mathrm g mathrm cm 3 g cm3 respectively Solution Verified Answered 2 years ago
Solved Gold Au Forms A Substitutional Solid Solution With Chegg
Answered step by step Gold forms a substitutional solid solution with silver Compute the weight percent of gold that must be added to silver to yield an alloy that contains 5 5 10 21 Au atoms per cubic centimeter The densities of pure Au and A g are 19 32 and 10 49 g c m 3 respectively

Gold Forms A Substitutional Solid Solution With Silver Compute The Number Of Gold Atoms Per

Solid Solution Intermetallic Compounds Substitutional Interstitial Ordered And
Gold Forms A Substitutional Solid Solution With Silver Compute
Gold forms a substitutional solid solution with silver Compute the number of gold atoms per cubic centimeter for a silver gold alloy that contains 25 wt Au and 75 wt Ag The densities of pure gold and silver are 19 32 and 10 49 g cm3 respectively The atomic weight of Au is 196 97 g mol
SOLVED Gold Forms A Substitutional Solid Solution
Chemical Engineering Chemical Engineering questions and answers 4 26 Gold forms a substitutional solid solution with silver Compute the number of gold atoms per cubic centimeter for a silver gold alloy that contains 10 wt Au and 90 wt Ag The densities of ure gold and silver are 19 32 and 10 49 g cm3 respectively
Answered step-by-step. Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic centimeter for a silver-gold alloy that contains 10 w t % A u and 90 w t % A g. The densities of pure gold and silver are 19.32 and 10.49 g …
Gold Forms A Substitutional Solid Solution With Silver Comp
Gold and silver both have the FCC crystal structure and Ag forms a substitutional solid solution for all concentrations at room temperature Compute the unit cell edge length for a 75 wt Au 25 wt Calculate the radius of silver atom in given that Ag has an FCC crystal structure a density of 10 5 gcm 3 and an atomic weight of 107 87

SOLVED Iron And Vanadium Both Have The BCC Crystal Structure And V Forms A Substitutional Solid

Solved 4 20 Molybdenum Forms A Substitutional Solid Solution With 1 Answer Transtutors
Gold Forms A Substitutional Solid Solution With Silver
Chemical Engineering Chemical Engineering questions and answers 4 26 Gold forms a substitutional solid solution with silver Compute the number of gold atoms per cubic centimeter for a silver gold alloy that contains 10 wt Au and 90 wt Ag The densities of ure gold and silver are 19 32 and 10 49 g cm3 respectively
Substitutional solid solutions are formed by melting elements into liquid form mixing them together then allowing the mixture to cool and solidify Elements will form substitutional
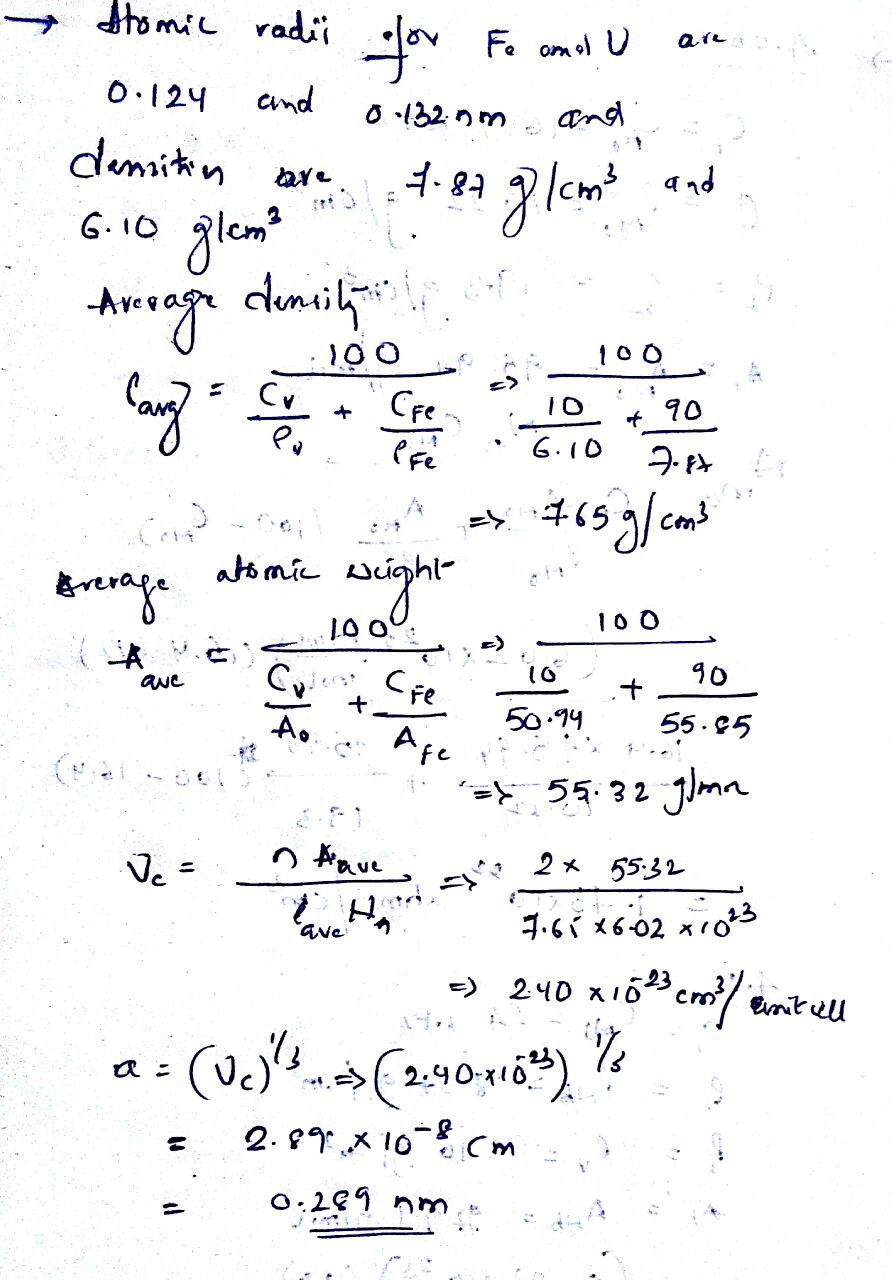
Solved 4 20 Molybdenum Forms A Substitutional Solid Solution With 1 Answer Transtutors
Solved Energy For Vacancy Formation eV atom 0 67 Chegg
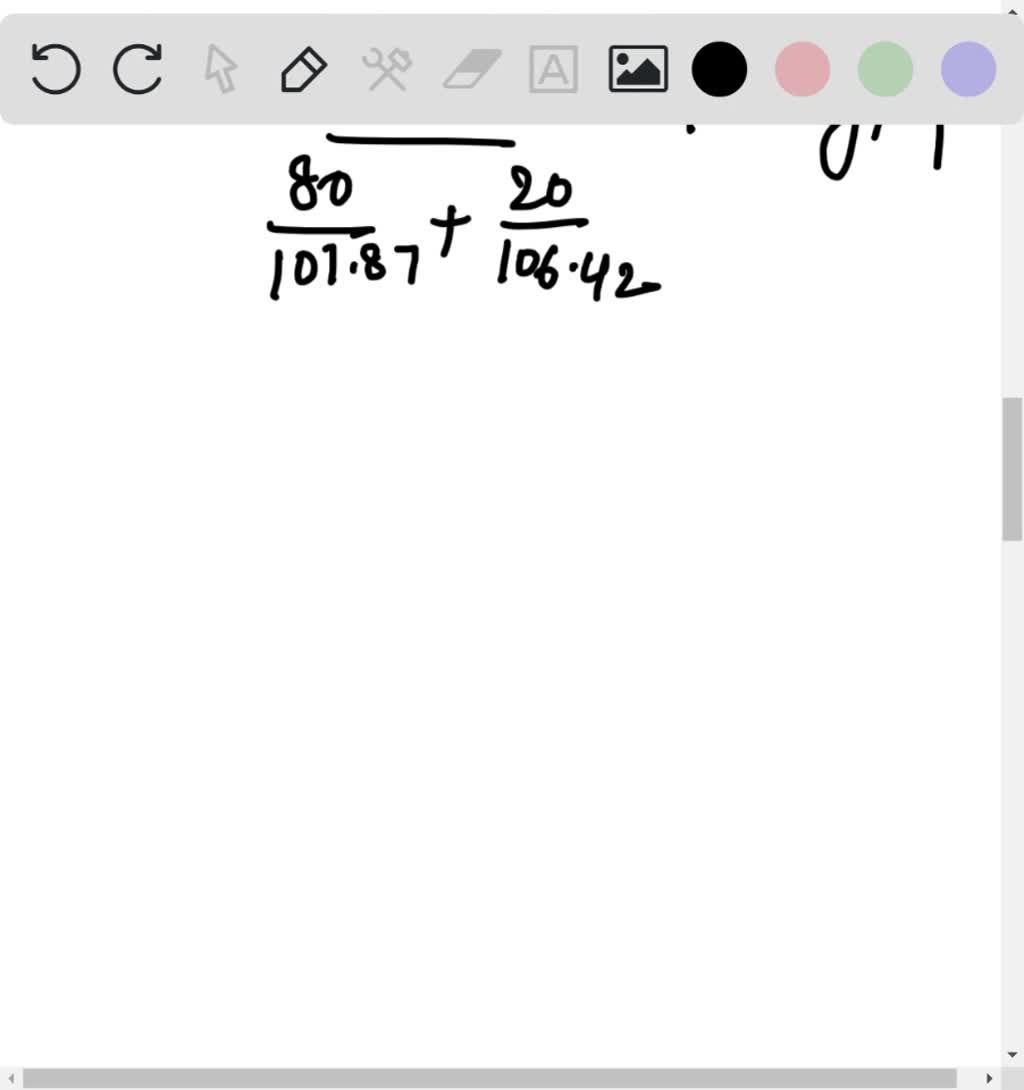
SOLVED Silver And Palladium Both Have The FCC Crystal Structure And Pd Forms A Substitutional
Solved Germanium Forms A Substitutional Solid Solution With Silicon Course Hero
Typs Of Alloys Tec science

